VERSIÓN EXCLUSIVA EN iOC2022 APP
← BACK
INDEX
Table of Contents
FOREWORD
Oncological disease is currently a real clinical challenge in terms of the diagnostic approach and the selection of the best treatment option, with a view to increasing patient survival and enhancing quality of life, but also in terms of coagulation and haemostasis management.
Oncological pathology, and particularly the tumour microenvironment, induces a natural state of hypercoagulability, which may present as simple analytical changes, asymptomatic prothrombotic states, manifest as extensive venous and arterial thromboembolic events, or increase the likelihood of bleeding complications associated with disseminated intravascular coagulation.
Venous thromboembolism (VTE) is an important cause of morbidity and mortality in cancer patients and is the second leading cause of death in this population, only surpassed by disease progression.
Cancer patients have a 4 to 7 times higher risk of developing VTE, and approximately 15% of them will present this diagnosis throughout the course of their disease, this figure being influenced by individual patient factors, the characteristics of the underlying disease, ongoing treatment, and other biomarkers.
VTE significantly worsens the prognosis of cancer patients, with a considerable impact on quality of life, as it requires prolonged anticoagulant therapy, with a significant risk of recurrence and potential haemorrhagic complications.
In this context, it is important to implement a strategy of active thromboprophylaxis by applying clinical risk scores to select patients who benefit most from these measures (clinical, mechanical methods or pharmacological options), in outpatients undergoing chemotherapy, but also in patients hospitalised in medical or surgical settings.
Despite the effort involved in this dynamic and given the high risk of developing VTE in this population, diagnosis of this entity should be considered a priority. Education of patients and healthcare professionals to value signs and symptoms is essential, to request appropriate diagnostic tests, safeguarding the range of asymptomatic episodes detected in routine imaging tests for disease assessment or staging, and for monitoring the response to oncological treatment.
In recent decades, low-molecular-weight heparins (LMWHs) have been considered the first-line pharmacological option for prophylaxis and treatment of cancer-associated thrombosis, as they have demonstrated superiority over vitamin K antagonists (VKAs) in reducing the risk of VTE recurrence without significantly increasing bleeding.
However, the rate of rethrombosis in this population remained high, and this may be partly explained by the limitations associated with LMWH, namely the need for parenteral administration and the high cost, which may lead to poor long-term adherence.
Currently, the prescription of direct oral anticoagulants (DOACs) as a treatment and prophylaxis strategy for VTE in oncology is the first therapeutic option, according to the main international guidelines.
Prescribing an DOAC in cancer patients is not straightforward, since despite the clear advantages associated with it, there are significant limitations to its use, and it should be avoided in patients at high risk of bleeding, particularly those with active mucosal lesions (gastrointestinal or genitourinary luminal), a history of recent haemorrhage, thrombocytopenia, severe renal or hepatic insufficiency, the presence of potential pharmacological interactions, in extreme body weight situations, or absence of a patent oral route, during nausea or vomiting secondary to antineoplastic therapies.
The approach to VTE treatment in cancer patients is therefore a constant and dynamic challenge, and it is essential to select the most appropriate pharmacological class, taking into account the severity of the thromboembolic event and its location, the underlying oncological pathology and the patient’s inherent characteristics. The appropriate period of treatment should never be overlooked, which should be maintained while the cancer is active, but should be subject to periodic medical evaluation, with assessment of clinical conditions and analytical parameters, and serial estimation of thrombotic and haemorrhagic risk.
Therefore, the bleeding risk should not be underestimated, not only in relation to anticoagulant therapy, but also secondary to oncological pathology, namely due to tumour infiltration of blood vessels, resulting from antineoplastic therapies (chemotherapy, radiotherapy, immunotherapy or surgical approach) and reflecting systemic changes associated with disease progression, such as liver metastasis or bone marrow infiltration.
Numerous local or systemic therapies are available to address this entity, depending on the likely site or focus of bleeding, such as haemostatic radiotherapy techniques, endoscopic procedures and vascular embolization methodologies.
With specific regard to haemorrhage associated with anticoagulant therapy, specific reversal agents are widely available, and antidotes for the main pharmacological classes are still being implemented, which will allow rapid reversal of the anticoagulant effect with assured haemostasis.
In view of this reality, it is essential to establish hospital protocols that allow a targeted and standardised approach to supportive therapies for cancer patients, always sharing a multidisciplinary approach to guidance and treatment, which will undoubtedly improve clinical outcomes in medical oncology.
Ricardo Pinto
Hospital Assistant of Immuno-Hemotherapy
Hospital Centre Vila Nova de Gaia/Espinho
Cancer and Thrombosis Study Group (GESCAT)
1. PULMONARY THROMBOEMBOLISM
Author: Ricardo Jorge Teixeira Pinto , MD
Co-author: Diogo Augusto, MD
1.1 Introduction
- Venous Thromboembolism (VTE), presenting in the form of Deep Vein Thrombosis (DVT) or Pulmonary Thromboembolism (PTE), represents an entity of relevance in cancer patients, which occurs in up to 20% of patients undergoing antineoplastic treatment. [1]
- PTE is considered a common and life-threatening event with a four-fold increased risk of occurrence in cancer patients compared to the general population. [2]
- The proper diagnosis and treatment of this entity are essential for the prevention of recurrent thromboembolic events, associated with a substantial increase in morbidity/mortality. [3]
1.2 Symptoms
Diagnosing PTE through clinical signs and symptoms is challenging, as they do not have enough sensitivity or specificity to diagnose or exclude pathology in most clinical situations. [4-7] Pulmonary auscultation as part of the physical exam usually does not provide diagnostic information. [5]
The most common signs or symptoms in the presentation are:
- Dyspnea at rest (50%) or with exertion (27%)
- Pleuritic chest pain (39%)
- Extremity oedema suggestive of DVT (24%)
Other signs or symptoms that may be present:
- Cough without haemoptysis (23%)
- Respiratory distress (16%)
- Substernal chest pain (15%)
- Dizziness (12%)
- Diaphoresis (12%)
Some manifestations are less common, but may be the initial presentation of PTE with hemodynamic impact, so it is important to identify:
- Cough with haemoptysis (8%)
- Syncope (6%)
- Shock (systolic blood pressure <90 mmHg or cyanosis) [5,7]
It is important to recognize that PTE shares signs and symptoms with other pathologies, such as pneumonia, pneumothorax, acute coronary syndrome or thoracic aortic dissection, so other causes should always be excluded. [4]
1.3 Aetiology
- PTE is defined as a block of the pulmonary arteries by a blood clot. [4-7
- Virchow described in the 19th century the pathophysiology of VTE that includes three variables: vascular statism, endothelial damage and hypercoagulability, situations improved by the nature of oncological pathology. [5.7]
- VTE of the lower extremities is more likely to embolize (15-32%) and cause PTE, while DVT of the twin veins is rarely embolized to the pulmonary vessels. However, it can, in about 33% of cases, progress to more proximal veins and increase the potential for embolization. Upper extremity DVT rarely (6%) presents with PTE. [5.7]
- After embolization of its point of origin, the thrombus, through the vena cava and the right chambers of the heart, can reach the pulmonary arteries, and can lodge in the main arterial bifurcation, depending on its dimensions, and establish severe hemodynamic compromise or lead to death. It can, on the other hand, fragment and reach the peripheral pulmonary arteries, causing pulmonary infarction with associated pain. [5.7]
PTE can be classified as:
- Massive PTE – Systolic arterial shock or systolic blood pressure <90 mmHg for more than 15 minutes requiring ionotropic support, absence of heart rate or bradycardia of <40 beats per minute.
- Sub massive PTE – No changes in blood pressure, but with right ventricular dysfunction (confirmed by imaging or elevation of cardiac markers).
- Low-risk PTE – Hemodynamic stability cardiac biomarkers without right ventricular dysfunction. [9]
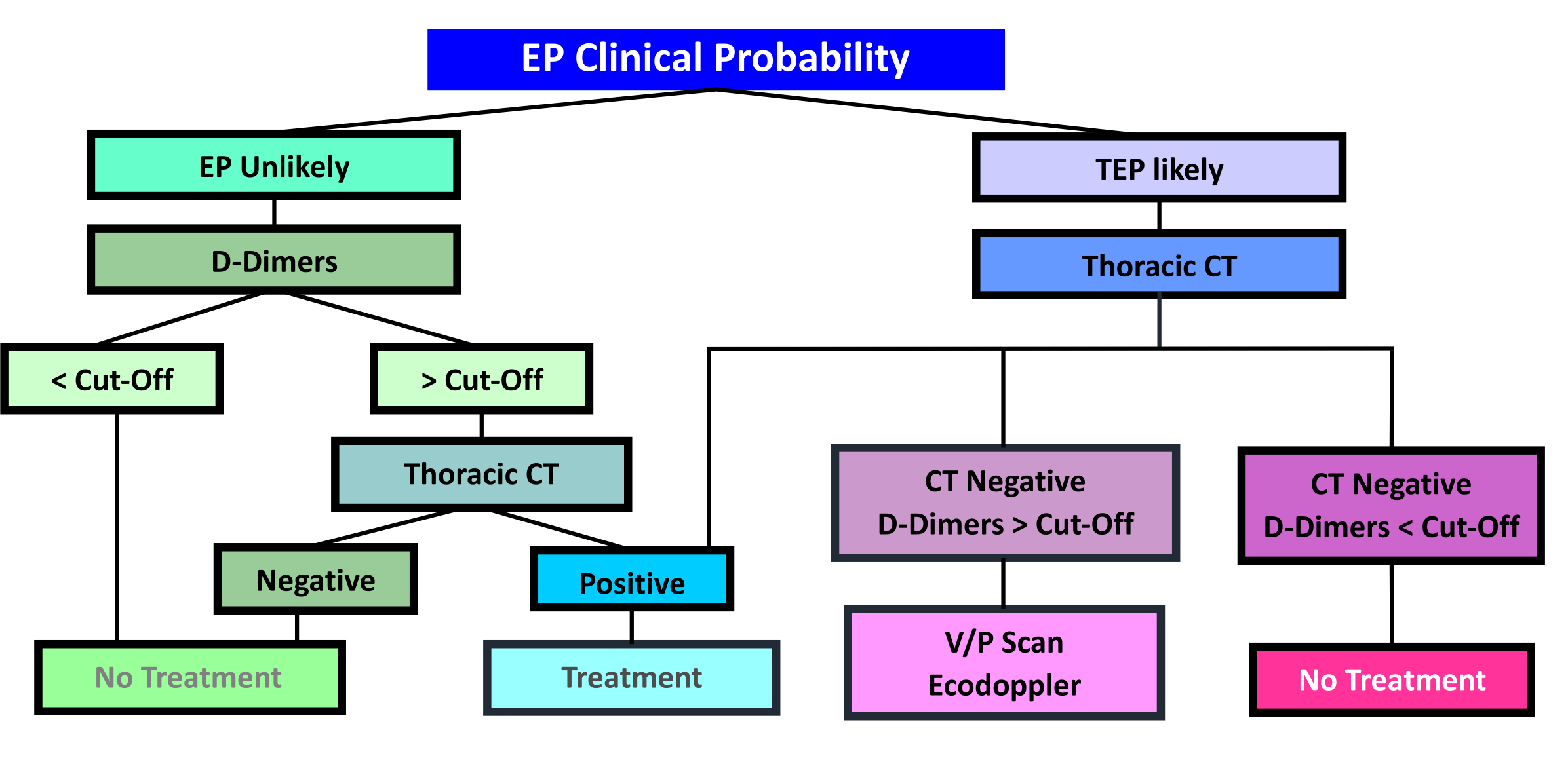
1.4 Diagnostic strategy
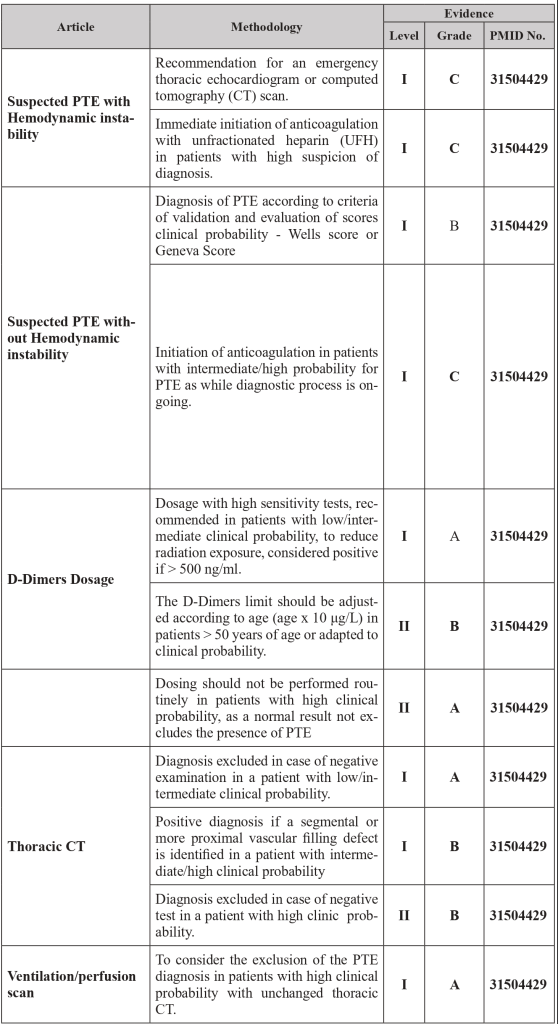
1.5 Risk Scores
| Legend: PE – Pulmonary Embolism | DVT- Deep venous thrombosis
1.6 Diagnostic Algorithm
1.7 Therapeutic Strategy
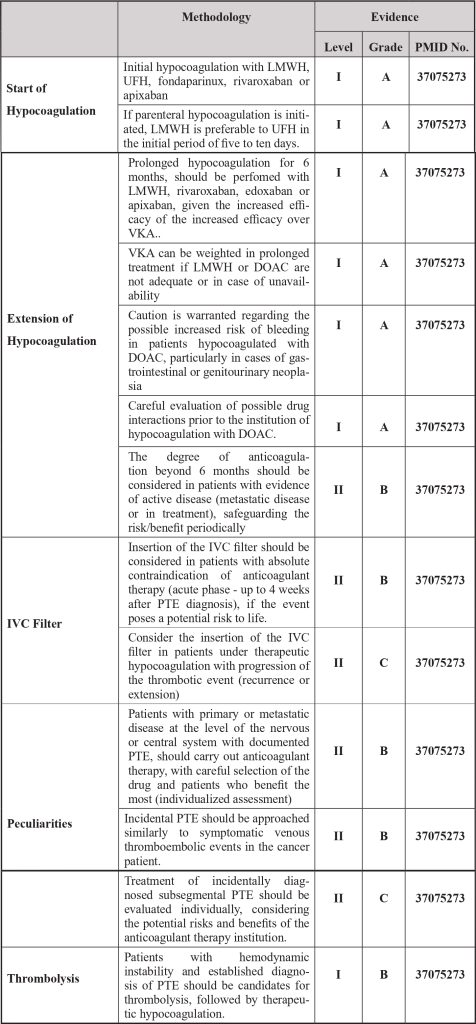
| Legend: IVC – Inferior Vena Cava
1.8 Pharmacotherapy
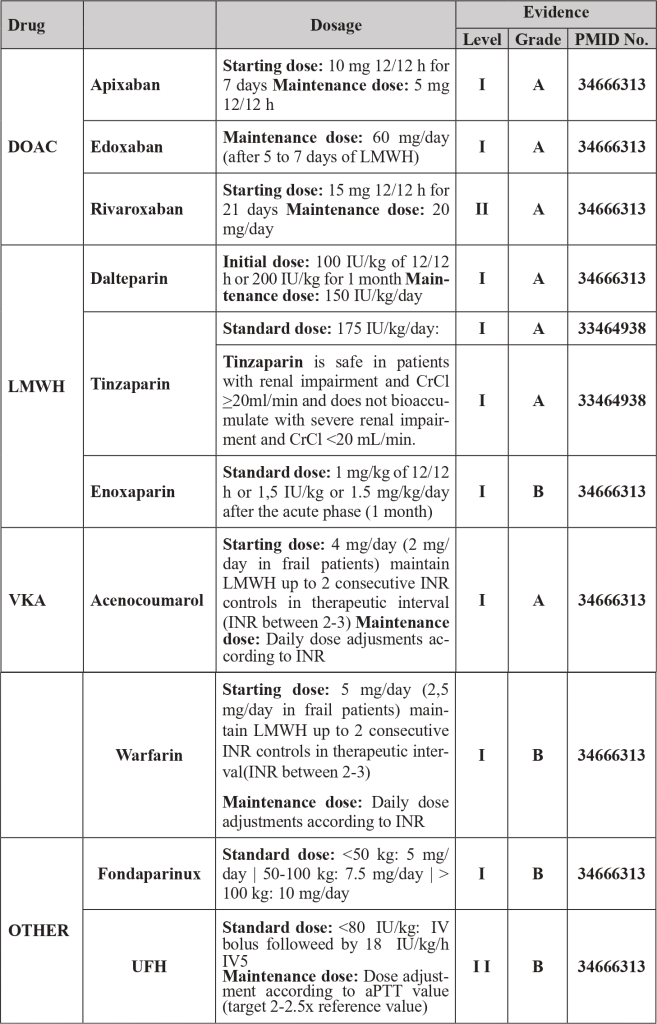
| Legend: DOAC – Direct Oral Anticoagulant | INR – International Normalized Ratio | VKA – Vitamin K Antagonists | LMWH – Low molecular weight heparin | UFH – Unfractionated heparin
- Treatment of PTE with direct oral anticoagulants (DOACs) requires dose adjustment in patients with renal impairment with creatinine clearance < 50 mL/min and are contraindicated if creatinine clearance < 15 mL/min and/or if platelet counts < 50×10⁹/L. [1.2]
- When low molecular weight heparins (LMWH) are used, the therapeutic dose of enoxaparin and dalteparin should be optimized in patients with creatinine clearance between 15-30 mL/min, Tinzaparin therapeutic dose can be administered in patients with creatinine clearance > 20mL/min. In patients with thrombocytopenia between 20-50×10⁹/L LMWH dosage should also be optimized. If platelet counts are lower, pharmacological hypocoagulation is contraindicated (in patients at high thrombotic risk consider transfusion of platelet concentrates to target counts > 50×10⁹/L to be able to administer therapeutic doses of LMWH). [1-3]
- To evaluate therapeutic efficacy or associated complications, consider dosing of DOAC concentration according to time of administration or monitoring of anti-Xa activity in patients receiving LMWH. [3]
1.9 Clinical Trials
| CLOT | Agnes Y.Y. Lee et al, Low molecular weight heparin versus coumarin for the prevention of recurrent venous thromboembolism in cancer patients, N Engl J Med 2003; 349: 146-153
| ONCENOX | Steven R Deitcher et al, Secundary prevention of venous thromboembolic event in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period, Clin Appl Thromb Hemost 2006, 4:389-96
| CATCH | Agnes Y.Y. Lee et al, CATCH: A randomized clinical trial comparing long-term tinzaparin versus warfarin for treatment of acute venous thromboembolism in cancer patients, BMC cancer 2013; 13:284
| Selectd-D | Young A et al, Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism: results of select-d Pilot Trial, Blood 2017; 130:625
| Hokusai VTE Cancer | Raskob GE et al, Edoxaban for the treatment of cancer-associated venous thromboembolism, N Engl J Med 2018; 378: 615-24
| Caravaggio | Giancarlo Agnelli, M.D. et al, Apixaban for the treatment of venous thromboembolism associated with cancer, N Engl J Med 2020; 382: 1599-1607
References
- Stockler M. R. et al, ASCO updated recommendations for preventing and treating VTE in adults with cancer, Ann Intern Med., 2020
- Michael B. et al, Cancer-Associated Venous Thromboembolic Disease, version 2.2021, NCCN Clinical Practice Guidelines in Oncology, J Natl Compr Canc Netw, 2021
- Stavros V. et al, ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS), European Heart Journal, 2020
- Huisman M. V., et al, Pulmonary Embolism, Nature Vol. 4, No. 18028, 2018
- Essien E., et al, Pulmonary Embolism, Medical Clinics of North America Vol. 103, No. 3, 2019
- Toplis E., Mortimore G., The Diagnosis and Management of Pulmonary Embolism, British Journal of Nursing Vol. 29, No. 1, 2020
- Pollack C. V., et al., Clinical Characteristics, Management, and Outcomes of Patients Diagnosed with Acute Pulmonary Embolism in the Emergency Department, Initial Report of EMPEROR, Journal of the American College of Cardiology Vol. 57, No. 6, 2011
- Turetz M., et al, Epidemiology, Pathophysiology, and Natural History of Pulmonary Embolism, Seminars in Interventional Radiology Vol. 35 No. 2, 2018
- Jaff M. R., et al, Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension – A Scientific Statement From the American Heart Association, American Heart Association, 2011 * Version 2. 2023
2. DEEP VEIN THROMBOSIS
Author: Inés Pintor, MD
Co-author: Joana Liz-Pimenta, MD
2.1 Definition and Epidemiology
|PMID 24939044; PMID 33275332; PMID 29703467; PMID 15564173; PMID 23908465; PMID 11861986; PMID 30402189|
- Deep vein thrombosis (DVT) is defined as the development of a blood clot in the veins, that most commonly occurs in the lower limbs (in 90% of the cases) and rarely affects veins in the upper limbs, abdomen, or brain.
- It is important to distinguish between the terms thrombosis and embolism, which is defined as dislodgement of the clot from the blood vessel where it was developed and getting stuck in another location, generally in the lungs, causing a pulmonary embolism (PE). Rarely PE can the develop in the absence of a DVT.
- Venous thromboembolism (VTE) includes DVT and PE. Patients with cancer have a higher risk of initial (four to sevenfold) and recurrent VTE (threefold) when compared to the general population, resulting in considerable morbidity and mortality. Making VTE the second cause of death in cancer patients. The risk of arterial thromboembolism is also higher in this population. Also, these patients have a two-fold higher risk of anticoagulation-associated bleeding. These complications occur due to the effects and interactions of the tumour.
- The cumulative incidence of venous thrombosis in cancer patients varies between 1-8% and it is rising. Besides, it is estimated that 20-30% of all initial VTE are cancer related.
- The most common presentation of DVT is leg swelling and the gold standard diagnostic method is duplex venous scanning, due to its high sensitivity and specificity.
- DVT and PE are the most common preventable causes of hospital death. It is important to identify patients who are most likely to benefit from pharmacologic prophylaxis and the effective treatment to reduce recurrence and mortality. The most important goal of thromboprophylaxis is to prevent complications and death (mostly fatal PE).
2.2 Symptoms and signs
|PMID 24939044; PMID 30402189; PMID 15564173; PMID: 27913509; PMID 23908465; PMID 17692901; PMID 18223291|
- VTE can be divided into symptomatic or incidental. Classical symptoms of DVT include swelling (80%), pain (75%), alteration in sensitivity and change in the temperature or colour of the limb, which becomes blue or reddish (26%). PE presents with shortness of breath, chest pain, palpitations, or collapse.
- There are a limited number of studies comparing the clinical presentation of DVT in patients with and without cancer. Bilateral DVT is more common in patients with cancer than in noncancer patients.
- Iliofemoral thrombosis may present with Phlegmasia cerulea dolens, an uncommon but potentially life-threatening complication. It presents with marked swelling of the limb, with pain and cyanosis. The massive limb swelling may be associated with arterial thrombosis, gangrene, amputation and even death.
- Postphlebitic syndrome is a state of chronic venous insufficiency due the loss of venous valvular function during the reorganization of the thrombus. It develops in 20-50% of patients with DVT, even when effective anticoagulant therapy was used. Clinical manifestations may include chronic leg pain with activity limitation, swelling and leg ulcers.
- VTE may be the first sign of cancer. Eight percent of idiopathic VTE will have cancer diagnosed in the first 12 months after VTE. Therefore, occult cancer should be excluded in the case of idiopathic VTE.
2.3 Aetiology
|PMID: 8173368; PMID 16284987; PMID 33570602; PMID 23908465; PMID 15564173; PMID 19381022; PMID: 22859911; PMID: 16145406; PMID: 19720906|
- The causes of thrombosis are summarized in Virchow´s triad, which includes stasis of blood, alteration in the composition of blood and changes in the vessel wall. The risk of VTE in cancer is increased in the first 3-6 months after diagnosis with further increases in cancer progressions It is the result of factors related to cancer, patients, treatment, and biomarkers:
- Hypercoagulability (tumours increase production of thrombin, tissue factor, factor VIII, D-Dimers, p-selectin and fibrinogen, increasing thrombogenic potential).
- Compression and invasion of the tumour to adjacent vessels.
- Type of cancer: primary tumour location, histologic type and molecular alterations have different risks. (The highest risk cancer are pancreas and gastric, followed by urologic tumours (except prostate), gynaecologic tumours, central nervous system (CNS) and lung. Hematologic cancer such as lymphoma and myeloma also have increased risk, but the pathophysiology is not entirely the same.
- Metastatic disease and high-stage cancer.
- Advanced age.
- Obesity.
- Prior history of venous thrombosis.
- Comorbidities (≥3 comorbid conditions).
- Anaemia, thrombocytosis, and leucocytosis
- Ethnicity (highest risk for African Americans and lowest for Asians).
- Hospitalization and prolonged immobility.
- Therapy: surgery (the risk of 90-day postoperative VTE is twice as high as in noncancer patients), chemotherapy (annual incidence: 11 – 20%, highest for platinum agents and gemcitabine), hormonal therapy (especially tamoxifen), targeted treatment agents (a major role for anti-VEGFRs and CDK4/6 inhibitors), thalidomide/lenalidomide, radiotherapy, red blood cell transfusions, erythropoietin-stimulating agents, and central venous catheters.
- Prothrombotic mutations (cancer patients with factor V Leiden have a twofold increased risk of VTE compared with noncarriers with cancer).
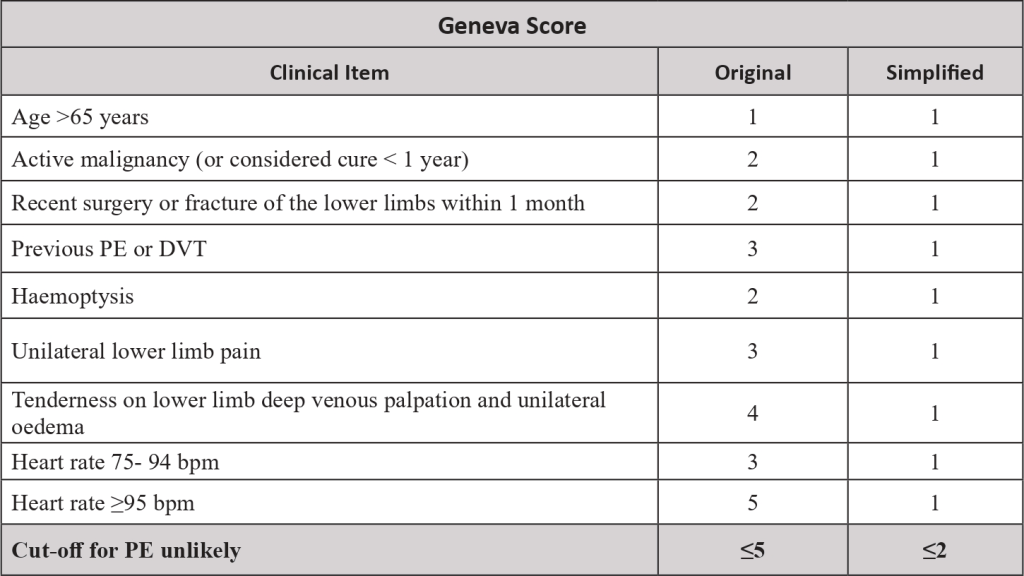
- From the study of these risk factors, risk scores were created, to predict which patient would be at increased risk of VTE. The most validated score is the Khorana score.
2.4 Diagnosis
|PMID 11861986; PMID 9308616; PMID 8667510; PMID 15564173; PMID 17112931; PMID 11686356; PMID: 18558426; PMID: 31155730; PMID 2202232|
- In oncologic patients, there must be a high index of suspicion for the presence of VTE, especially when there is present some of the already mentioned risk factors. Early diagnosis and treatment are essential given thrombus embolization and PE potential.
- The diagnosis of DVT requires a combination of clinical assessment, pre-test probability and objective diagnostic testing.
- A physical examination should be performed to look for dilated superficial veins, unilateral swelling with inflammatory signs (warmth, tenderness, or erythema) and pain along the course of the involved veins. However, these signs and symptoms lack specificity.
- Limb swelling is the most common indication for duplex venous scan, but it is not predictive of DVT. Some studies have shown that a discrepancy of less than 2 cm in the calf circumference of the involved and the normal limb predicted the absence of DVT in 93% of patients.
- Studies have shown an association between PE, history of malignancy or previous DVT and a positive result on the duplex scan. A study found that in patients with PE confirmed by pulmonary angiogram or ventilation-perfusion scan, the incidence of acute DVT detected by duplex scan was 43%. Though, if the diagnosis of PE was clinical, the incidence of DVT was only 10%.
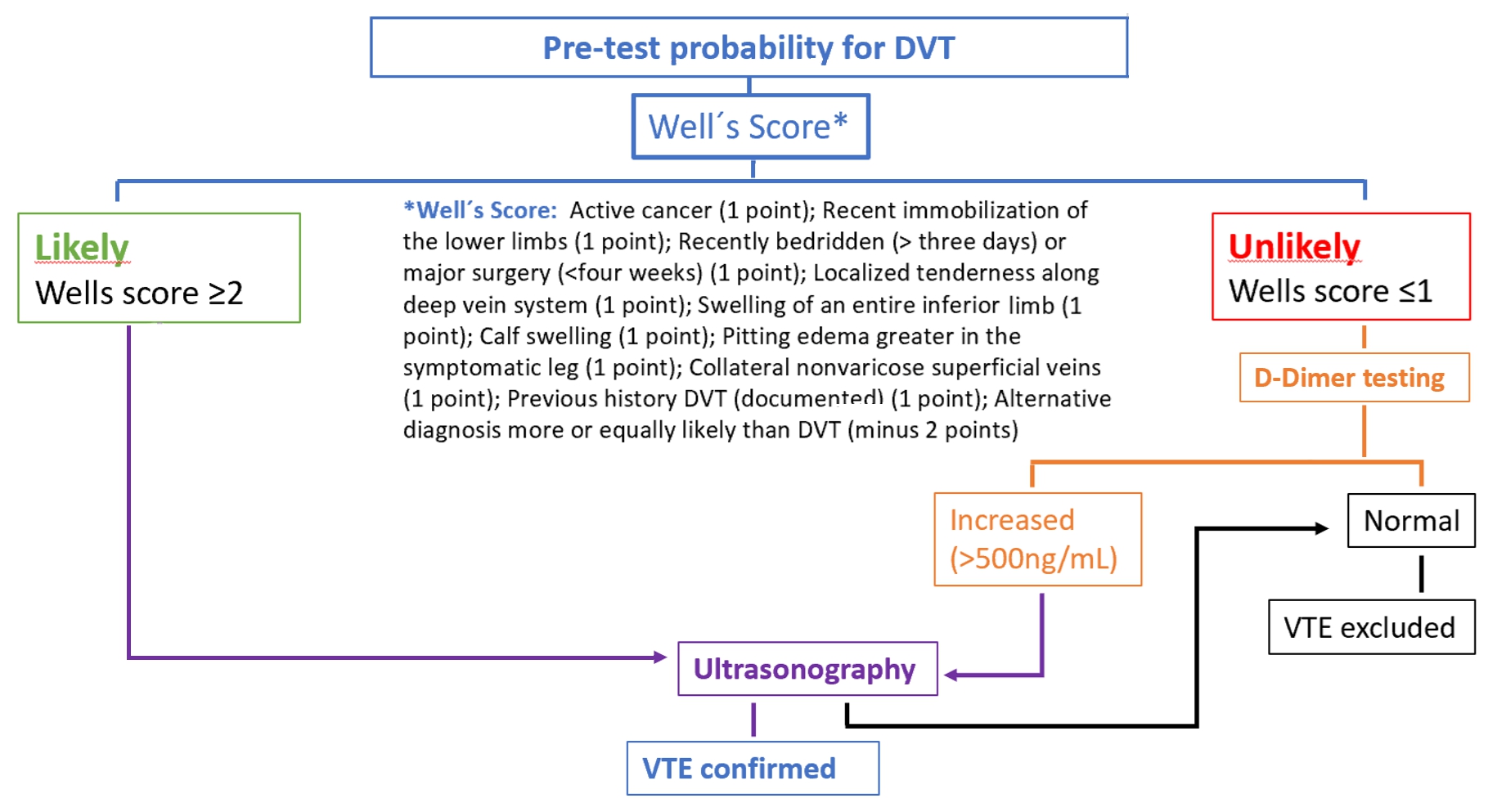
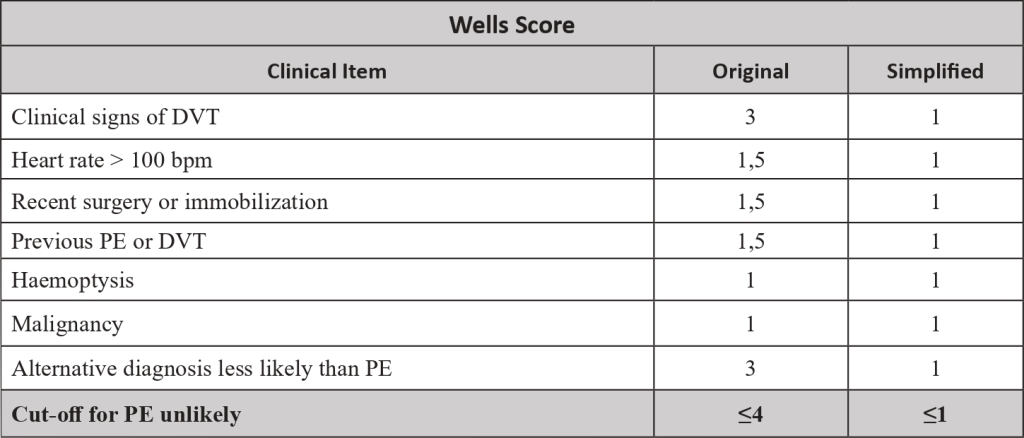
- The pre-test probability is made by a clinical decision risk assessment model that classifies the probability of DVT. The best-studied scoring system is the modified Wells Score, which includes the following components.
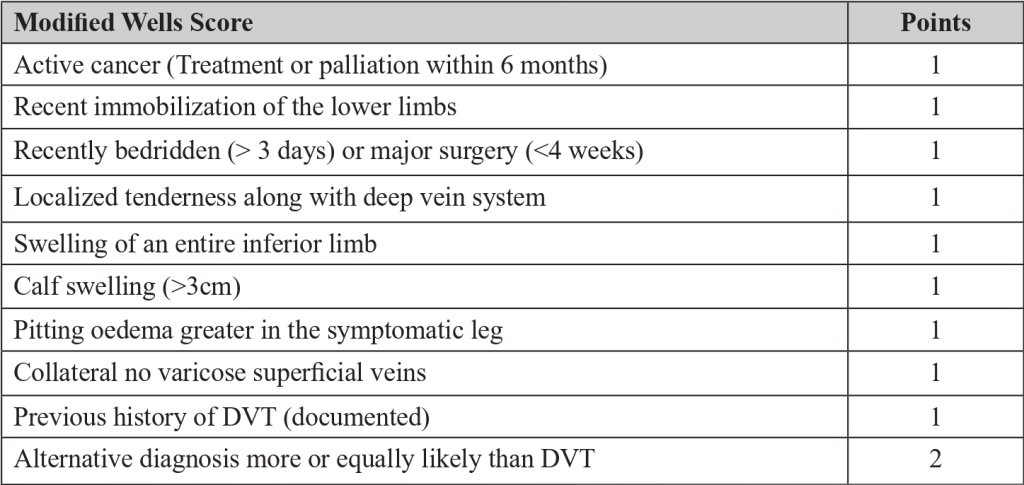
Two level DVT Wells socre
- This score divides patients into “likely” (score ≥2) or “unlikely” (score ≤1) to have DVT.
- Patients classified as “unlikely” should be referred for D-dimer testing. DVT is excluded if the value is normal (<500ng/mL). If the value is increased (>500ng/mL), ultrasonography of the inferior limb should be performed. If ultrasonography is negative, DVT is excluded.
- Patients classified as “likely” should be referred for lower limb ultrasonography. In these patients, the D-dimer value cannot be reliably used to exclude DVT. If ultrasonography is negative, DVT is excluded.
- In a study , the sensitivity, specificity, positive predictive value and negative predictive value of a low pre-test probability Wells score in combination with a negative D-dimer result were 99%, 33%, 29% and 99%, respectively. In cancer patients, D-dimer does not work so well as a discriminator factor, because they may be risen due to cancer proinflammatory state.
- Duplex venous ultrasound is the gold standard for diagnosis of DVT, with an overall sensitivity and specificity around 95% and 100%, respectively.
Click to see image
2.5 Therapy
PMID: 31381464; PMID 34878173; PMID: 29363105; PMID: 26210891; PMID: 29231094; PMID: 29746227; PMID: 29506866; PMID 33570602; PMID 30482768; PMID: 16670137; PMID: 27344439|
- In cancer patients the treatment and prophylaxis are based on anticoagulation.
- Absolute contraindications for the use of anticoagulation include:
- Active major, serious, or potentially life-threatening bleeding is not reversible with medical or surgical intervention.
- Uncontrolled malignant hypertension.
- Severe coagulopathy or platelet dysfunction (severe thrombocytopenia: < 20000/mL) or inherited bleeding disorder.
- High-risk invasive procedure in a critical site (e.g.: lumbar puncture, spinal anaesthesia, epidural catheter placement).
- Concurrent use of potent P-glycoprotein or CYP3A4 (inhibitors or inducers (DOAC specific).
- Relative contraindications for the use of anticoagulation include intracranial or spinal lesion at elevated risk for bleeding; active GI ulceration at high risk of bleeding; active but non–life-threatening bleeding; intracranial bleeding (< 4 weeks); recent high-risk surgery or bleeding event; persistent thrombocytopenia (<50.000/mL).
- In the case of thrombocytopenia is important to access whether the anticoagulation should be reduced or stopped or if a platelet transfusion should be done.
- Anticoagulation showed benefit in incidental VTE, CNS thrombosis and palliative settings, but more studies are ongoing in these populations.
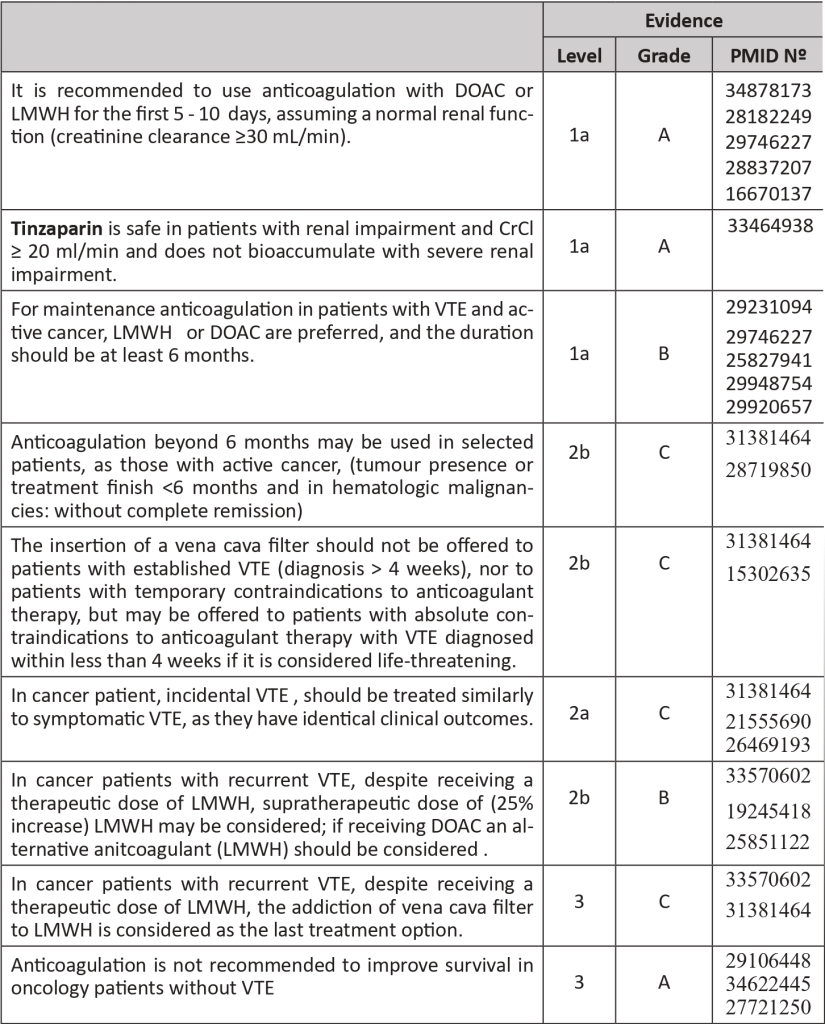
- In a patient with DVT, anticoagulation may be started with Low Molecular Weight Heparin (LMWH), and Direct Oral Anticoagulants (DOACs). In special circumstances, Unfractionated Heparin (UFH) or Fondaparinux may also be used. The initial period of treatment includes 5 – 10 days. Only two DOACs (Rivaroxaban and Apixaban) have been approved for the treatment of VTE in the initial period (Edoxaban can be used for maintenance treatment) Tinzaparin is the LMWH with better results in cancer patient trials. UFH may be the first option for patients with renal impairment. Fondaparinux should be considered for patients with heparin-induced thrombocytopenia background.
- Meta-analyses studies confirm the superiority of LMWH relatively to Vitamin K antagonist (VKA) in reducing the risk of VTE recurrence in cancer patients, as well as adverse effects. Therefore, VKA should not be used routinely in cancer patients.
- In the meta-analysis, DOACs had a lower risk of VTE, but a higher risk of major bleeding compared with LMWH, with no significant difference in mortality. In patients with an increased risk of bleeding (use of antiplatelet agents, renal or hepatic impairment, thrombocytopenia, history of gastrointestinal bleeding, gastrointestinal or genitourinary cancers, and polypharmacy), LMWH is safer. There is limited information on DOAC use in patients with primary malignancy or metastasis of the CNS as well as its use in catheter-related thrombosis.
- For VTE treatment besides anticoagulation, a vena cava filter can be an option for very selected patients.
- In cancer patients with VTE, the standard duration of anticoagulation is 6 months. Nonetheless, duration beyond this time or indefinite anticoagulation may be beneficial in reducing the recurrence but not the mortality. Treatment for only three months can be evaluated in cases of incidental and peripheral VTE or catheter-related thrombosis. Anticoagulation can be stopped when patients do not present VTE at imaging exams nor clinical VTE signs and when they no longer have active cancer. A periodic revaluation should be done every 3-6 months, looking for risk of thrombosis and bleeding, cancer status and prognosis, treatments, comorbidities, costs and one of the most crucial factors: patient preferences and values.
- Recurrence may occur in patients already in use of standard-dose anticoagulation (5-7%). In these cases, treatment compliance should be assessed, as well as any mechanical compression caused by the tumour or heparin-induced thrombocytopenia. If the patient is under DOAC it should be changed to LMW. If already in LMWH should be considered a 25% increase in the dosage. Adding a vena cava filter to LMWH is considered the last line of treatment.
- Age is a risk factor for bleeding. However, anticoagulation should be offered to older patients if there are no contraindications. Anticoagulants should be used with caution in special populations, such as patients with renal impairment, fall risk, cognitive impairment, poor functional status, weight extremes or without family or medical support.
- Renal impairment increases the risk of bleeding, especially in cancer patients. Limited data suggest that LMWH may accumulate with therapeutic doses if the creatinine clearance is inferior to 30 mL/min, increasing at least two-fold the risk of bleeding when compared to patients with normal renal function. In patients with cancer and renal impairment, UFH and VKA are wiser choices for initial and long-term therapy, respectively.
The recommended anticoagulants for treatment are:
- LMWH at least during first 5 days: (Evidence: 1a, A, PMID: 28182249, 29363105, 31381464, 19147527, 16082604):
- Dalteparin 100 U/kg every 12 hours or 200 U/kg once daily for one month and then 150 U/Kg once daily.
- Tinzaparin 175 U/kg once daily.
- Enoxaparin 1 mg/kg every 12 hours or 1.5 mg/kg once daily.
- DOACs:
- Rivaroxaban 15mg orally every 12 hours for 21 days, followed by 20mg once daily (Evidence: 1a, A, PMID: 29746227, 31381464).
- Apixaban 10mg orally twice daily for 7 days, followed by 5mg twice daily (Evidence 1a, A, PMID: 32223112, 28837207, 31381464).
- Edoxaban 60mg once daily, after 5-7 days of LMWH.
Regarding thromboprophylaxis:
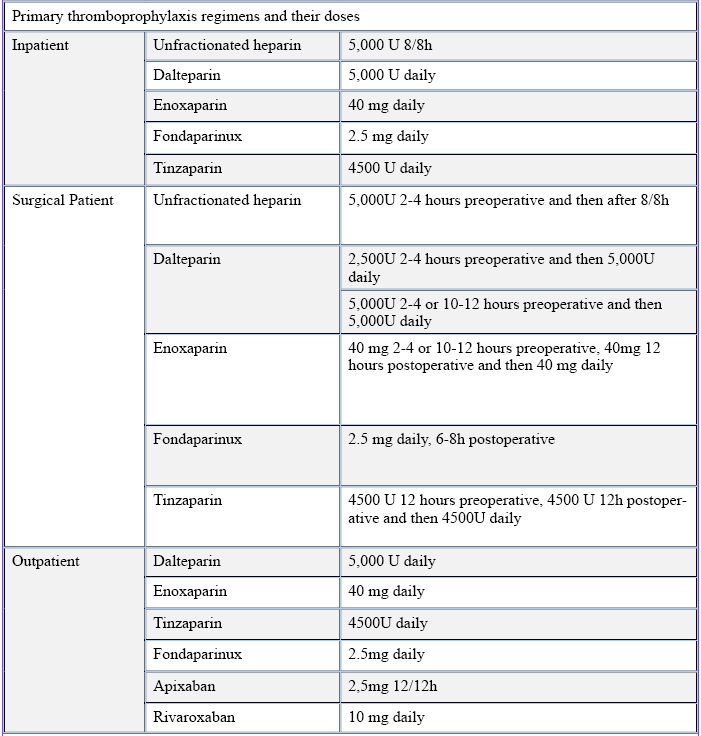
- Nowadays, cancer outpatients with a Khorana score ≥2 and no contraindications should start thromboprophylaxis for 6 months, with LMWH or DOAC (Apixaban or Rivaroxaban) – same criteria for the choice in VTE treatment applied, but reduced doses: Tinzaparin 4500 UI daily, Enoxaparin 40mg daily, Dalteparin 5000UI daily, Apixaban 2,5mg twice a day and Rivaroxaban 10mg daily.
- Patients with Multiple Myeloma under thalidomide or lenalidomide with chemotherapy and/or dexamethasone also should be under thromboprophylaxis.
- In hospitalized patients, thromboprophylaxis with LMWH is the treatment of choice if active cancer and no contraindications, during the period of hospitalization. .
- In surgical cancer patients, thromboprophylaxis with LMWH is also the treatment of choice and is recommended in cases of major surgery, for a minimum of 7-10 days, and in abdominal/pelvic surgery for 4 weeks.
References
- PMID 24939044: Thachil J. Deep vein thrombosis. Hematology. 2014 Jul;19(5):309-10. doi: 10.1179/1024533214Z.000000000284. PMID: 24939044.
- PMID 33275332: Streiff MB, Abutalib SA, Farge D, Murphy M, Connors JM, Piazza G. Update on Guidelines for the Management of Cancer-Associated Thrombosis. Oncologist. 2021 Jan;26(1):e24-e40. doi: 10.1002/onco.13596. Epub 2020 Dec 4. PMID: 33275332; PMCID: PMC7794170.
- PMID 29703467: Lyman GH, Culakova E, Poniewierski MS, Kuderer NM. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb Res. 2018 Apr;164 Suppl 1:S112-S118. doi: 10.1016/j.thromres.2018.01.028. PMID: 29703467.
- PMID 15564173: Yap KP, McCready DR. Deep vein thrombosis and malignancy: a surgical oncologist’s perspective. Asian J Surg. 2004 Jul;27(3):249-54. doi: 10.1016/S1015-9584(09)60045-2. PMID: 15564173.
- PMID 23908465: Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013 Sep 5;122(10):1712-23. doi: 10.1182/blood-2013-04-460121. Epub 2013 Aug 1. PMID: 23908465.
- PMID 11861986: Lee YM, Ting AC, Cheng SW. Diagnosing deep vein thrombosis in the lower extremity: correlation of clinical and duplex scan findings. Hong Kong Med J. 2002 Feb;8(1):9-11. PMID: 11861986.
- PMID 30402189: Chaochankit W, Akaraborworn O. Phlegmasia Cerulea Dolens with Compartment Syndrome. Ann Vasc Dis. 2018 Sep 25;11(3):355-357. doi: 10.3400/avd.cr.18-00030. PMID: 30402189; PMCID: PMC6200621.
- PMID 27913509: Kahn SR. The post-thrombotic syndrome. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):413-418. doi: 10.1182/asheducation-2016.1.413. PMID: 27913509; PMCID: PMC6142466.
- PMID 17692901: Agnelli G, Verso M, Ageno W, Imberti D, Moia M, Palareti G, Rossi R, Pistelli R; MASTER investigators. The MASTER registry on venous thromboembolism: description of the study cohort. Thromb Res. 2008;121(5):605-10. doi: 10.1016/j.thromres.2007.06.009. Epub 2007 Aug 10. PMID: 17692901.
- PMID 18223291: Imberti D, Agnelli G, Ageno W, Moia M, Palareti G, Pistelli R, Rossi R, Verso M; MASTER Investigators. Clinical characteristics and management of cancer-associated acute venous thromboembolism: findings from the MASTER Registry. Haematologica. 2008 Feb;93(2):273-8. doi: 10.3324/haematol.11458. Epub 2008 Jan 26. PMID: 18223291.
- PMID 8173368: Nordström M, Lindblad B, Anderson H, Bergqvist D, Kjellström T. Deep venous thrombosis and occult malignancy: an epidemiological study. BMJ. 1994 Apr 2;308(6933):891-4. doi: 10.1136/bmj.308.6933.891. PMID: 8173368; PMCID: PMC2539869.
- PMID 16284987: Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005 Dec 15;104(12):2822-9. doi: 10.1002/cncr.21496. PMID: 16284987.
- PMID 33570602: Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, Leavitt AD, Lee AYY, Macbeth F, Morgan RL, Noble S, Sexton EA, Stenehjem D, Wiercioch W, Kahale LA, Alonso-Coello P. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021 Feb 23;5(4):927-974. doi: 10.1182/bloodadvances.2020003442. Erratum in: Blood Adv. 2021 Apr 13;5(7):1953. PMID: 33570602; PMCID: PMC7903232.
- PMID 19381022: Obitsu Y, Shigematsu H. [Deep vein thrombosis in patients with cancer]. Gan to Kagaku ryoho. Cancer & Chemotherapy. 2009 Apr;36(4):535-539. PMID: 19381022.
- PMID 22859911: Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275. Epub 2012 Jul 31. PMID: 22859911; PMCID: PMC3409130.
- PMID 16145406: Smith JA Jr. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. J Urol. 2005 Oct;174(4 Pt 1):1300. doi: 10.1097/01.ju.0000178536.63739.af. PMID: 16145406.
- PMID 19720906: Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009 Oct 10;27(29):4839-47. doi: 10.1200/JCO.2009.22.3271. Epub 2009 Aug 31. PMID: 19720906; PMCID: PMC2764392.
- PMID 9308616: Criado E, Burnham CB. Predictive value of clinical criteria for the diagnosis of deep vein thrombosis. Surgery. 1997 Sep;122(3):578-83. doi: 10.1016/s0039-6060(97)90131-8. PMID: 9308616.
- PMID 8667510: Fowl RJ, Strothman GB, Blebea J, Rosenthal GJ, Kempczinski RF. Inappropriate use of venous duplex scans: an analysis of indications and results. J Vasc Surg. 1996 May;23(5):881-5; discussion 885-6. doi: 10.1016/s0741-5214(96)70251-3. PMID: 8667510.
- PMID 17112931: Subramaniam RM, Snyder B, Heath R, Tawse F, Sleigh J. Diagnosis of lower limb deep venous thrombosis in emergency department patients: performance of Hamilton and modified Wells scores. Ann Emerg Med. 2006 Dec;48(6):678-85. doi: 10.1016/j.annemergmed.2006.04.010. Epub 2006 Jun 9. PMID: 17112931.
- PMID 11686356: Constans J, Nelzy ML, Salmi LR, et al. Clinical prediction of lower limb deep vein thrombosis in symptomatic hospitalized patients. Thrombosis and Haemostasis. 2001 Oct;86(4):985-990. PMID: 11686356.
- PMID 18558426: Carrier M, Lee AY, Bates SM, Anderson DR, Wells PS. Accuracy and usefulness of a clinical prediction rule and D-dimer testing in excluding deep vein thrombosis in cancer patients. Thromb Res. 2008;123(1):177-83. doi: 10.1016/j.thromres.2008.05.002. Epub 2008 Jun 16. PMID: 18558426.
- PMID 31155730: Kruger PC, Eikelboom JW, Douketis JD, Hankey GJ. Deep vein thrombosis: update on diagnosis and management. Med J Aust. 2019 Jun;210(11):516-524. doi: 10.5694/mja2.50201. Epub 2019 Jun 2. PMID: 31155730.
- PMID 2202232: Habscheid W, Höhmann M, Wilhelm T, Epping J. Real-time ultrasound in the diagnosis of acute deep venous thrombosis of the lower extremity. Angiology. 1990 Aug;41(8):599-608. doi: 10.1177/000331979004100803. PMID: 2202232.
- PMID 31381464: Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, Gates LE, Kakkar AK, Levine MN, Liebman HA, Tempero MA, Lyman GH, Falanga A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020 Feb 10;38(5):496-520. doi: 10.1200/JCO.19.01461. Epub 2019 Aug 5. PMID: 31381464.
- PMID 34878173: Kahale LA, Matar CF, Hakoum MB, Tsolakian IG, Yosuico VE, Terrenato I, Sperati F, Barba M, Schünemann H, Akl EA. Anticoagulation for the initial treatment of venous thromboembolism in people with cancer. Cochrane Database Syst Rev. 2021 Dec 8;12(12):CD006649. doi: 10.1002/14651858.CD006649.pub8. PMID: 34878173; PMCID: PMC8653422.
- PMID 29363105: Hakoum MB, Kahale LA, Tsolakian IG, Matar CF, Yosuico VE, Terrenato I, Sperati F, Barba M, Schünemann H, Akl EA. Anticoagulation for the initial treatment of venous thromboembolism in people with cancer. Cochrane Database Syst Rev. 2018 Jan 24;1(1):CD006649. doi: 10.1002/14651858.CD006649.pub7. Update in: Cochrane Database Syst Rev. 2021 Dec 8;12:CD006649. PMID: 29363105; PMCID: PMC6389339.
- PMID 26210891: Posch F, Königsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015 Sep;136(3):582-9. doi: 10.1016/j.thromres.2015.07.011. Epub 2015 Jul 17. PMID: 26210891; PMCID: PMC7311195.
- PMID 29231094: Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang TF, Yeo E, Zhang G, Zwicker JI, Weitz JI, Büller HR; Hokusai VTE Cancer Investigators. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-624. doi: 10.1056/NEJMoa1711948. Epub 2017 Dec 12. PMID: 29231094.
- PMID 29746227: Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, MacCallum P, Kakkar A, Hobbs FDR, Petrou S, Dale J, Poole CJ, Maraveyas A, Levine M. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-2023. doi: 10.1200/JCO.2018.78.8034. Epub 2018 May 10. PMID: 29746227.
- PMID 29506866: Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb Res. 2019 Jan;173:158-163. doi: 10.1016/j.thromres.2018.02.144. Epub 2018 Mar 2. PMID: 29506866; PMCID: PMC6119655.
- PMID 30482768: Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, Linkins LA, Rodner SB, Selleng S, Warkentin TE, Wex A, Mustafa RA, Morgan RL, Santesso N. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018 Nov 27;2(22):3360-3392. doi: 10.1182/bloodadvances.2018024489. PMID: 30482768; PMCID: PMC6258919.
- PMID 16670137: Lim W, Dentali F, Eikelboom JW, Crowther MA. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med. 2006 May 2;144(9):673-84. doi: 10.7326/0003-4819-144-9-200605020-00011. PMID: 16670137.
- PMID 27344439: Woodruff S, Feugère G, Abreu P, Heissler J, Ruiz MT, Jen F. A post hoc analysis of dalteparin versus oral anticoagulant (VKA) therapy for the prevention of recurrent venous thromboembolism (rVTE) in patients with cancer and renal impairment. J Thromb Thrombolysis. 2016 Nov;42(4):494-504. doi: 10.1007/s11239-016-1386-8. PMID: 27344439; PMCID: PMC5040733.
3. COAGULOPATHY
Author: João Ricardo Cordeiro, MD
Co-author: Clara Maria Dias Pinto, MD
Co-author: Ana Isabel Paiva Santos, MD
3.1 Introduction
PMID 15925818: PMID 22777060 25054907 PMID 24862149
- Cancer induces an acquired state of hypercoagulability characterized by an activation of the coagulation cascade, whose clinical manifestations range from an asymptomatic prothrombotic state, only with analytical changes, to the appearance of thrombosis of large vessels, which can culminate in haemorrhagic events associated with disseminated intravascular coagulation.
- The pathogenesis of cancer-associated coagulopathy is complex and multifactorial associated with multiple cancer-related risk factors, such as its anatomical location but also with the patient and treatment. Among the mechanisms associated with neoplasia we find the expression of haemostatic proteins by tumour cells (eg.: Tissue factor), the production of inflammatory cytokines, proangiogenic factors, among others.
- Forms of presentation:
- Superficial migratory thrombophlebitis – Trousseau syndrome.
- Deep vein thrombosis and pulmonary thromboembolism.
- Nonbacterial thrombotic endocarditis – Marathic endocarditis.
- Disseminated intravascular coagulation.
- Thrombotic Microangiopathy – Thrombotic Thrombocytopenic Purpura.
- Arterial thrombosis.
3.2 Thromboprophylaxis in Oncology Patients
Inpatient Thromboprophylaxis
PMID: 10477777; PMID: 15289368; PMID: 16439370; PMID: 22077144; PMID: 23388003; PMID: 24384102; PMID 30638566; PMID: 31381464; PMID: 31492632; PMID: 33861298
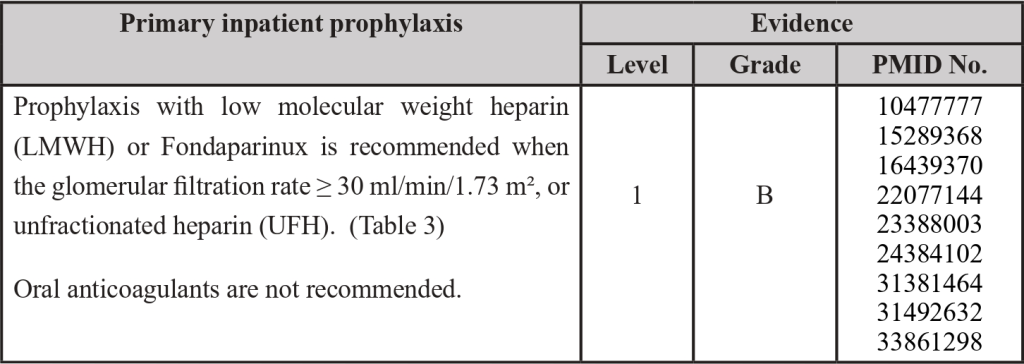
3.3 Thromboprophylaxis in Surgical Patients
PMID: 11442521; PMID: 16881934; PMID: 20456751; PMID: 24253138; PMID: 24966161; PMID: 26386868; PMID: 26887853; PMID: 27591773; PMID: 27591773; PMID: 27849664; PMID: 28577378; PMID: 28846822; PMID: 29097086; PMID: 29397103; PMID: 30027281; PMID: 31381464
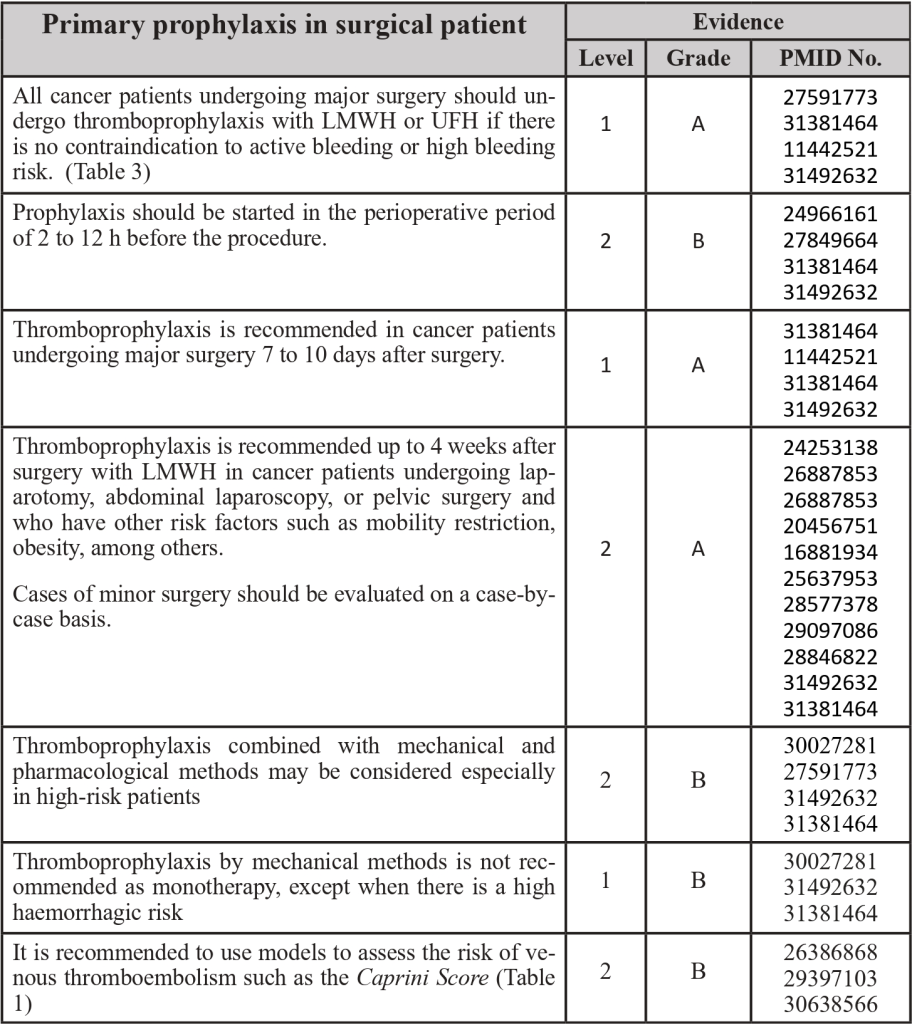
Table 1 – Caprini Score
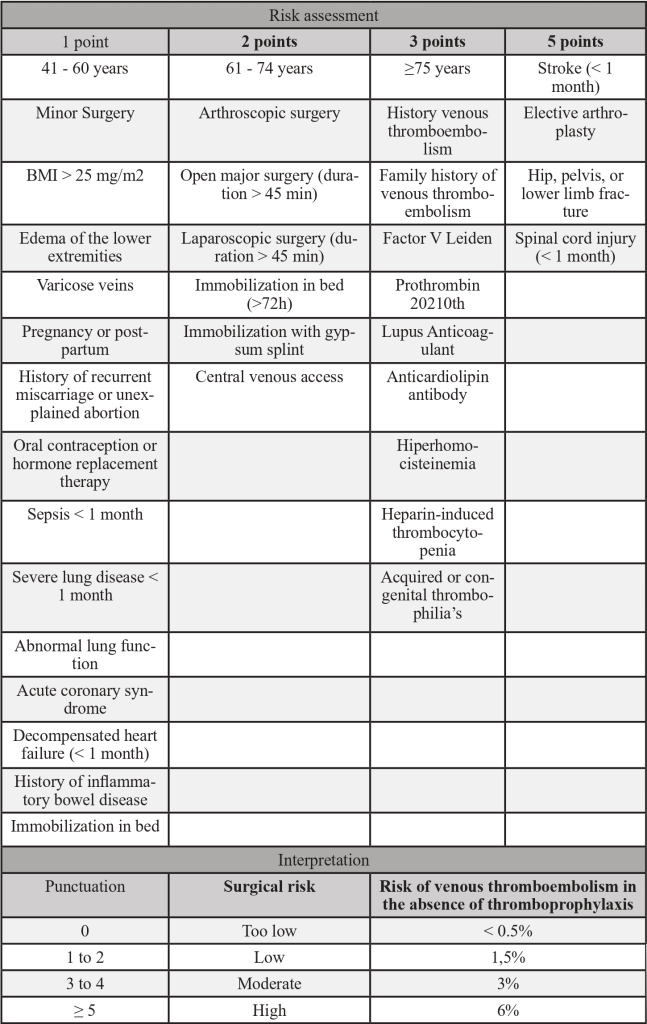
Adapted from PMID 30638566: Golemi I, Salazar Adum JP, Tafur A, Caprini J. Venous thromboembolism prophylaxis using the Caprini score. Disease-a-Month [Internet]. 2019;65(8):249–98. Available from: https://doi.org/10.1016/j.disamonth.2018.12.005
3.4 Outpatient thromboprophylaxis
PMID: 22100906; PMID: 24665264; PMID: 25162954; PMID: 25987694; PMID: 26738412; PMID: 26963028; PMID: 27906452; PMID: 28139259; PMID: 28240823; PMID: 28402864; PMID: 28949077; PMID: 29733498; PMID: 30511879; PMID: 30786186
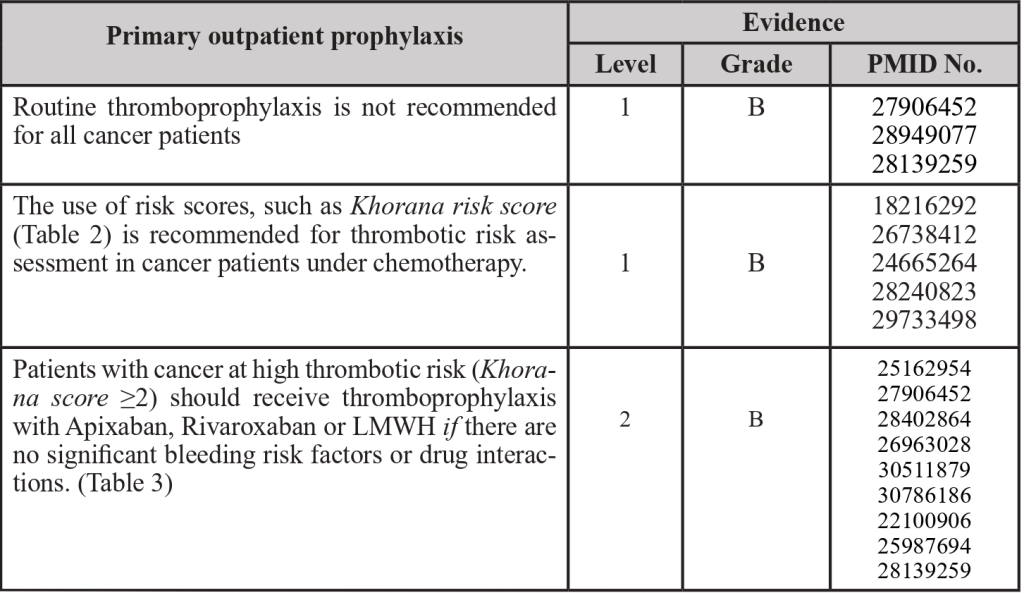
Table 2- Khorana’s score
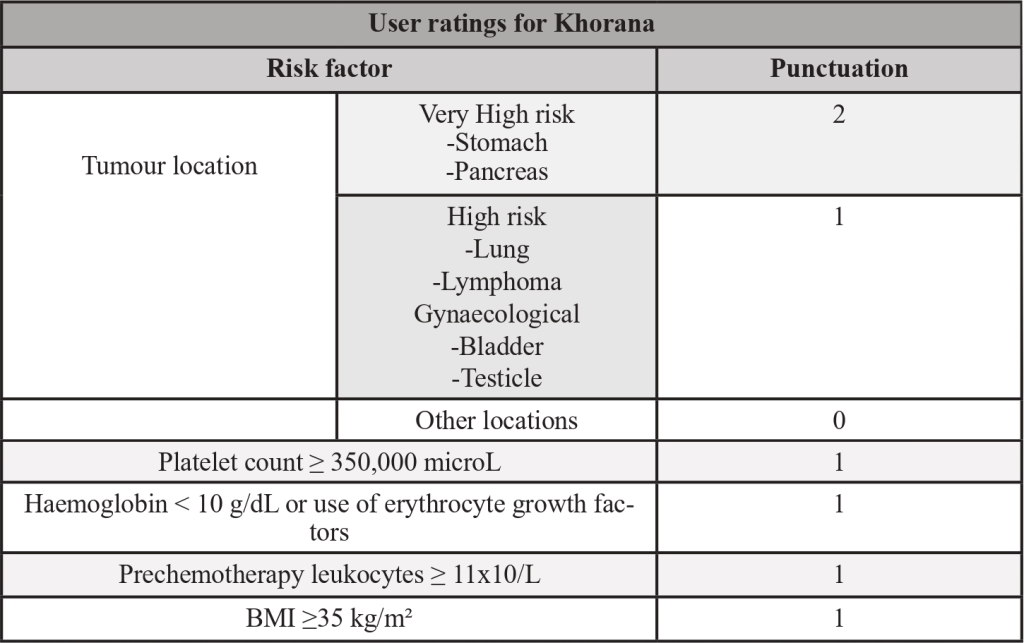
Adapted from: PMID 31381464: Key, N. S., Khorana, A. A., Kuderer, N.M., Bohlke, K., Lee, A. Y. Y., Arcelus, J. I., Wong, S. L., Balaban, E. P., Flowers, C. R., Francis, C. W., Gates, L. E., Kakkar, A. K., Levine, M. N., Liebman, H. A., Tempero, M. A., L
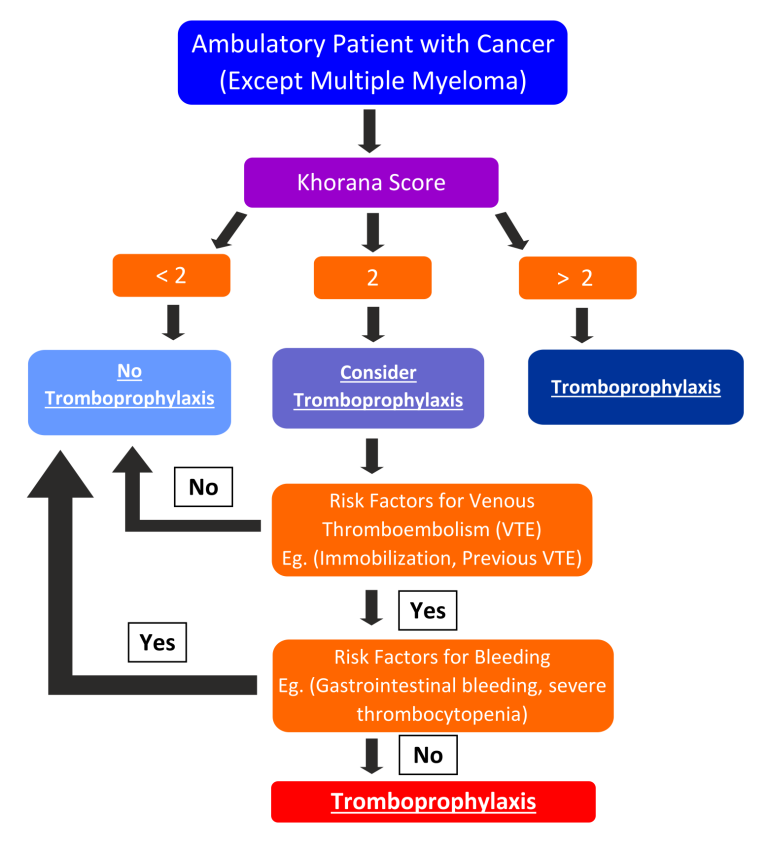
Click to see image
Figure 1 – Algorithm for primary thromboprophylaxis in cancer patients (except multiple myeloma)
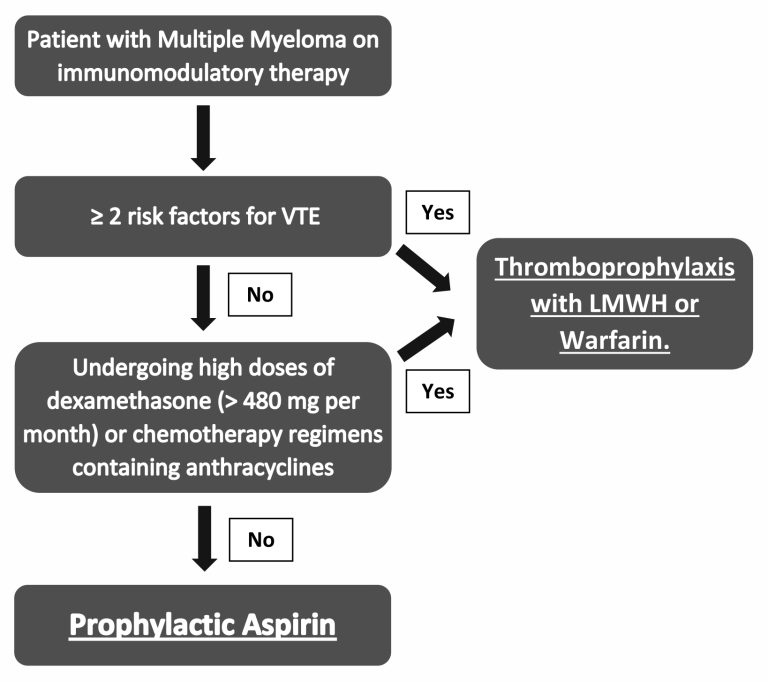
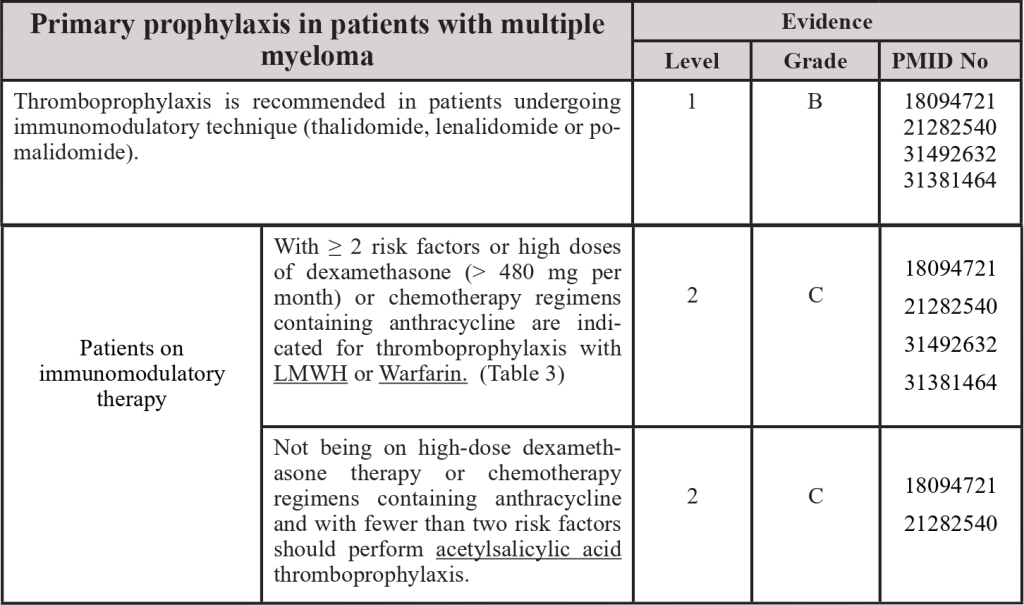
3.5 Thromboprophylaxis in the patient with multiple myeloma
PMID: 18094721; PMID: 21282540; PMID: 31492632; PMID: 31381464
Risk factors for venous thromboembolism:
- Previous venous thromboembolism.
- Hereditary thrombophilia.
- Central venous catheter or pacemaker support.
- Heart disease (e.g., heart failure, a history of stent, coronary bypass);
- Diabetes Mellitus.
- Acute infection.
- Immobilization.
- Use of erythropoietin.
- Chronic kidney disease.
- BMI ≥ 30 kg/m².
Figure 2 – Algorithm for thromboprophylaxis in patients with multiple myeloma
Click to see image
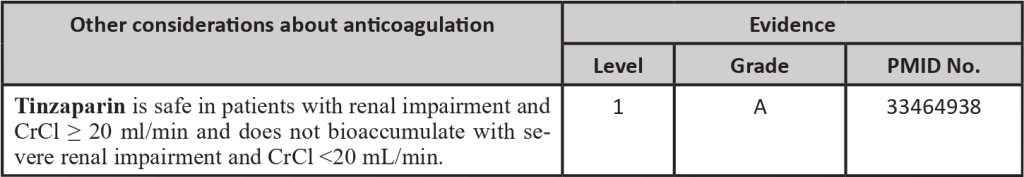
Table 3 – Primary thromboprophylaxis regimens and their doses
Adapted from: PMID 31381464: Key, N. S., Khorana, A. A., Kuderer, N.M., Bohlke, K., Lee, A. Y. Y., Arcelus, J. I., Wong, S. L., Balaban, E. P., Flowers, C. R., Francis, C. W., Gates, L. E., Kakkar, A. K., Levine, M. N., Liebman, H. A., Tempero, M. A., Lyman, G. H., &
3.6 Absolute and relative contraindications to anticoagulation
PMID 31381464
- Absolute contraindications:
ºActive, severe, and life-threatening bleeding.
ºSevere uncontrolled hypertension.
ºHemorrhagic diathesis.
ºPersistent and severe thrombocytopenia (< 20,000/ml);
ºInvasive procedures (e.g., lumbar puncture, spinal anaesthesia). - Relative contraindications:
ºIntracranial or spinal cord injury with high risk of bleeding.
ºActive ulceration of the gastrointestinal tract with high risk of bleeding.
ºActive, but not life-threatening bleeding.
ºBleeding in the central nervous system in the past 4 weeks.
ºRecent high-risk surgery or recent bleeding event.
ºPersistent thrombocytopenia (< 50,000/ml)
References
- PMID 10477777: Samama, M.M., Cohen, A. T., Darmon, J.-Y., Desjardins, L., Eldor, A., Janbon, C., Leizorovicz, A., Nguyen, H., Olsson, C.-G., Turpie, A. G., & Weisslinger, N. (1999). A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in critically ill medical patients. New England Journal of Medicine, 341(11), 793–800. https://doi.org/10.1056/nejm199909093411103
- PMID 11442521: Mismetti, P., Laporte, S., Darmon, J. Y., Buchmüller, A., & Decousus, H. (2001). Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. British Journal of Surgery, 88(7), 913–930. https://doi.org/10.1046/j.0007-1323.2001.01800.x
- PMID 15289368: Samama, M.M., Cohen, A. T., Darmon, J.-Y., Desjardins, L., Eldor, A., Janbon, C., Leizorovicz, A., Nguyen, H., Olsson, C.-G., Turpie, A. G., & Weisslinger, N. (1999). A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in critically ill medical patients. New England Journal of Medicine, 341(11), 793–800. https://www.nejm.org/doi/full/10.1056/NEJM199909093411103
- PMID 15925818: Piccioli, A., Falanga, A., Baccaglini, U., Marchetti, M., & Prandoni, P. (2006). Cancer and venous thromboembolism. Seminars on Thrombosis and Hemostasis, 32(7), 694–699. https://doi.org/10.1055/s-2006-951297
- PMID 16439370: Cohen, A. T., Davidson, B. L., Gallus, A. S., Lassen, M. R., Prins, M. H., Tomkowski, W., Turpie, A. G. G., Egberts, J. F.M., & Lensing, A. W. A. (2006). Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in elderly acute medical patients: A randomized placebo-controlled trial. British Medical Journal, 332(7537), 325–327. https://doi.org/10.1136/bmj.38733.466748.7C
- PMID 16881934: Rasmussen, M. S., Jorgensen, L. N., Wille-Jørgensen, P., Nielsen, J. D., Horn, A., Mohn, A.C., Sømod, L., Olsen, B., Neergaard, K., Harvald, T., Hansen, H., & Pilsgaard, B. (2006). Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: an open-label multicenter randomized study. Journal of Thrombosis and Haemostasis, 4(11), 2384–2390. https://doi.org/10.1111/j.1538-7836.2006.02153.x
- PMID 18094721: Palumbo, A., Rajkumar, S. V., Dimopoulos, M. A., Richardson, P. G., San Miguel, J., Barlogie, B., Harousseau, J., Zonder, J. A., Cavo, M., Zangari, M., Attal, M., Belch, A., Knop, S., Joshua, D., Sezer, O., Ludwig, H., Vesole, D., Bladé, J., Kyle, R., … Hussein, M. A. (2008). Prevention of thalidomide-associated thrombosis and lenalidomide in myeloma. Leukemia, 22(2), 414–423. PMID https://doi.org/
- PMID 20456751: Kakkar, V. V., Balibrea, J. L., Martínez-González, J., & Prandoni, P. (2010). Extended bemiparin prophylaxis for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: The CANBESURE randomized study. Journal of Thrombosis and Haemostasis, 8(6), 1223–1229. https://doi.org/10.1111/j.1538-7836.2010.03892.x
- PMID 21282540: Palumbo, A., Cavo, M., Bringhen, S., Zamagni, E., Romano, A., Patriarca, F., Rossi, D., Gentilini, F., Crippa, C., Galli, M., Nozzoli, C., Ria, R., Marasca, R., Montefusco, V., Baldini, L., Elice, F., Callea, V., Pulini, S., Carella, A.M., … Boccadoro, M. (2011). Thromboprophylaxis with aspirin, warfarin, or enoxaparin in patients with multiple myeloma treated with thalidomide: a randomized phase III, open-label trial. Journal of Clinical Oncology, 29(8), 986–993. https://doi.org/10.1200/JCO.2010.31.6844
- PMID 22077144: Goldhaber, S. Z., Leizorovicz, A., Kakkar, A. K., Haas, S. K., Merli, G., Knabb, R.M., & Weitz, J. I. (2011). Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. New England Journal of Medicine, 365(23), 2167–2177. https://doi.org/10.1056/nejmoa1110899
- PMID 22100906: Maraveyas, A., Waters, J., Roy, R., Fyfe, D., Propper, D., Lofts, F., Sgouros, J., Gardiner, E., Wedgwood, K., Ettelaie, C., & Bozas, G. (2012). Gemcitabine versus gemcitabine plus thromboprophylaxis with dalteparin in pancreatic cancer. European Journal of Cancer, 48(9), 1283–1292. https://doi.org/10.1016/j.ejca.2011.10.017
- PMID 22777060: Young, A., Chapman, O., Connor, C., Poole, C., Rose, P., & Kakkar, A. K. (2012). Thrombosis and cancer. Nature Reviews Clinical Oncology, 9(8), 437–449. https://doi.org/10.1038/nrclinonc.2012.106
- PMID 23388003: Cohen, A. T., Spiro, T. E., Büller, H. R., Haskell, L., Hu, D., Hull, R., Mebazaa, A., Merli, G., Schellong, S., Spyropoulos, A.C., & Tapson, V. (2013). Rivaroxaban for thromboprophylaxis in seriously ill medical patients. New England Journal of Medicine, 368(6), 513–523. https://doi.org/10.1056/nejmoa1111096
- PMID 24253138: Vedovati, M.C., Becattini, C., Rondelli, F., Boncompagni, M., Camporese, G., Balzarotti, R., Mariani, E., Flamini, O., Pucciarelli, S., Donini, A., & Agnelli, G. (2014). A randomized study on prophylaxis of 1 week versus 4 weeks for venous thromboembolism after laparoscopic surgery for colorectal cancer. Annals of Surgery, 259(4), 665–669. https://doi.org/10.1097/SLA.0000000000000340
- PMID 24384102: Carrier, M., Khorana, A. A., Moretto, P., Le Gal, G., Karp, R., & Zwicker, J. I. (2014). Lack of evidence to support thromboprophylaxis in hospitalised medical patients with cancer. American Journal of Medicine, 127(1), 82-86.e1. https://doi.org/10.1016/j.amjmed.2013.09.015
- PMID 24665264: Hohl Moinat, C., Périard, D., Grueber, A., Hayoz, D., Magnin, J. L., André, P., Kung, M., & Betticher, D.C. (2014). Predictors of venous thromboembolic events associated with central venous port insertion in cancer patients. Journal of Oncology, 2014, 1–7. https://doi.org/10.1155/2014/743181
- PMID 24862149: Levi, M. (2014). Cancer-related coagulopathies. Thrombosis Investigation, 133 (SUPPL. 2), S70–S75. https://doi.org/10.1016/S0049-3848(14)50012-6
- PMID 24966161: Akl, E. A., Kahale, L. A., Sperati, F., Neumann, I., Labedi, N., Terrenato, I., Barba, M., Sempos, E. V., Muti, P., Cook, D., & Schünemann, H. (2014). Low molecular weight heparin versus unfractionated heparin for perioperative thromboprophylaxis in cancer patients. Cochrane Database of Systematic Reviews, 2014(6). https://doi.org/10.1002/14651858.CD009447.pub2
- PMID 25054907: Falanga, A., Russo, L., & Milesi, V. (2014). Cancer coagulopathy. Current opinion in hematology, 21(5), 423–429. https://doi.org/10.1097/MOH.0000000000000072
- PMID 25162954: Ben-Aharon, I., Stemmer, S.M., Leibovici, L., Shpilberg, O., Sulkes, A., & Gafter-Gvili, A. (2014). Low molecular weight heparin (LMWH) for primary thromboprophylaxis in patients with solid malignancies – Systematic review and meta-analysis. Acta Oncologica, 53(9), 1230–1237. https://doi.org/10.3109/0284186X.2014.934397
- PMID 25987694: Pelzer, U., Opitz, B., Deutschinoff, G., Stauch, M., Reitzig, P.C., Hahnfeld, S., Müller, L., Grunewald, M., Stieler, J.M., Sinn, M., Denecke, T., Bischoff, S., Oettle, H., Dörken, B., & Riess, H. (2015). Efficacy of prophylactic low molecular weight heparin for outpatients with advanced pancreatic cancer: results from the CONKO-004 trial. Journal of Clinical Oncology, 33(18), 2028–2034. https://doi.org/10.1200/JCO.2014.55.1481
- PMID 26386868: Hachey, K. J., Hewes, P. D., Porter, L. P., Ridyard, D. G., Rosenkranz, P., McAneny, D., Fernando, H.C., & Litle, V. R. (2016). Caprini’s risk assessment of venous thromboembolism allows selection for post-discharge prophylactic anticoagulation in patients with resectable lung cancer. Journal of Thoracic and Cardiovascular Surgery, 151(1), 37-44.e1. https://doi.org/10.1016/j.jtcvs.2015.08.039
- PMID 26738412: Posch, F., Riedl, J., Reitter, E.M., Kaider, A., Zielinski, C., Pabinger, I., & Ay, C. (2016). Hypercoagulability, venous thromboembolism and death in cancer patients: a multistate model. Thrombosis and hemostasis, 115(4), 817–826. https://doi.org/10.1160/TH15-09-0758
- PMID 26887853: Fagarasanu, A., Alotaibi, G. S., Hrimiuc, R., Lee, A. Y. Y., & Wu, C. (2016). Role of extended thromboprophylaxis after abdominal and pelvic surgery in cancer patients: a systematic review and meta-analysis. Annals of Surgical Oncology, 23(5), 1422–1430. https://doi.org/10.1245/s10434-016-5127-1
- PMID 26963028: Tun, N.M., Guevara, E., & Oo, T. H. (2016). Benefit and risk of primary thromboprophylaxis in outpatients with advanced pancreatic cancer receiving chemotherapy: a systematic review and meta-analysis of randomised controlled trials. Blood clotting and fibrinolysis, 27(3), 270–274. https://doi.org/10.1097/MBC.0000000000000413
- PMID 27591773: Alshehri, N., Cote, D. J., Hulou, M.M., Alghamdi, A., Alshahrani, A., Mekary, R. A., & Smith, T. R. (2016). Venous thromboembolism prophylaxis in patients with brain tumors undergoing craniotomy: a meta-analysis. Journal of Neuro-Oncology, 130(3), 561–570. https://doi.org/10.1007/s11060-016-2259-x
- PMID 27849664: Guo, Q., Huang, B., Zhao, J., Ma, Y., Yuan, D., Yang, Y., & Du, X. (2017). Perioperative pharmacological thromboprophylaxis in cancer patients: a systematic review and meta-analysis. Annals of Surgery, 265(6), 1087–1093. https://doi.org/10.1097/SLA.0000000000002074
- PMID 27906452: Rutjes, A. W. S., Porreca, E., Candeloro, M., Valeriani, E., & Di Nisio, M. (2020). Primary prophylaxis for venous thromboembolism in outpatient cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews, 2020(12). https://doi.org/10.1002/14651858.CD008500.pub5
- PMID 28139259: Khorana, A. A., Francis, C. W., Kuderer, N.M., Carrier, M., Ortel, T. L., Wun, T., Rubens, D., Hobbs, S., Iyer, R., Peterson, D., Baran, A., Kaproth-Joslin, K., & Lyman, G. H. (2017). Dalteparin thromboprophylaxis in cancer patients at high risk of venous thromboembolism: a randomized trial. Thrombosis Research, 151, 89–95. https://doi.org/10.1016/j.thromres.2017.01.009
- PMID 28240823: Patell, R., Rybicki, L., McCrae, K. R., & Khorana, A. A. (2017). Prediction of the risk of venous thromboembolism in hospitalized cancer patients: Usefulness of a risk assessment tool. American Journal of Hematology, 92(6), 501–507. https://doi.org/10.1002/ajh.24700
- PMID 28402864: Fuentes, H. E., Oramas, D.M., Paz, L. H., Casanegra, A. I., Mansfield, A. S., & Tafur, A. J. (2017). Meta-analysis on anticoagulation and prevention of thrombosis and mortality in patients with lung cancer. Thrombosis Research, 154, 28–34. https://doi.org/10.1016/j.thromres.2017.03.024
- PMID 28577378: Stensland, K. D., Katz, E. G., & Canes, D. (2017). Re: Long-duration enoxaparin decreases the rate of venous thromboembolic events after radical cystectomy compared to subcutaneous heparin for hospitalized patients alone: J. J. Pariser, S.M. Pearce, B.B. Anderson, V. T. Packiam, V. N. Prachand, N. D. Smith and G. D. Steinberg J Urol 2017; 197: 302–307. Journal of Urology, 198(3), 707–708. https://doi.org/10.1016/j.juro.2017.04.076
- PMID 28846822: Kim, B. J., Day, R. W., Davis, C. H., Narula, N., Kroll, M. H., Tzeng, C. W. D. & Aloia, T. A. (2017). Extended pharmacological thromboprophylaxis in liver oncological surgery is safe and effective. Journal of Thrombosis and Haemostasis, 15(11), 2158–2164. https://doi.org/10.1111/jth.13814
- PMID 28949077: Thein, K. Z., Yeung, S.C. J. & Oo, T. H. (2018). Primary thromboprophylaxis (PTPs) in outpatients with lung cancer receiving chemotherapy: systematic review and meta-analyses of randomised controlled trials (RCTs). Asia-Pacific Journal of Clinical Oncology, 14(3), 210–216. https://doi.org/10.1111/ajco.12770
- PMID 29097086: Schomburg, J., Krishna, S., Soubra, A., Cotter, K., Fan, Y., Brown, G., & Konety, B. (2018). Prolonged outpatient chemoprophylaxis reduces venous thromboembolism after radical cystectomy. Urological Oncology: Seminars and Original Research, 36(2), 77.e9-77.e13. https://doi.org/10.1016/j.urolonc.2017.09.029
- PMID 29397103: Sterbling, H.M., Rosen, A. K., Hachey, K. J., Vellanki, N. S., Hewes, P. D., Rao, S. R., Pinjic, E., Fernando, H.C., & Litle, V. R. (2018). The caprini risk model decreases the rates of venous thromboembolism in patients with thoracic cancer. Annals of Thoracic Surgery, 105(3), 879–885. https://doi.org/10.1016/j.athoracsur.2017.10.013
- PMID 29733498: Parker, A., Peterson, E., Lee, A. Y. Y., de Wit, C., Carrier, M., Polley, G., Tien, J. & Wu, C. (2018). Risk stratification for the development of venous thromboembolism in hospitalized patients with cancer. Journal of Thrombosis and Haemostasis, 16(7), 1321–1326. https://doi.org/10.1111/jth.14139
- PMID 30027281: Jung, Y. J., Seo, H. S., Park, C. H., Jeon, H.M., Kim, J. Il, Yim, H. W. & Song, K. Y. (2018). Incidence of venous thromboembolism and use of prophylaxis after gastrectomy among Korean patients with gastric adenocarcinoma: the protective randomized clinical trial. JAMA Surgery, 153(10), 939–946. https://doi.org/10.1001/jamasurg.2018.2081
- PMID 30511879: Carrier, M., Abou-Nassar, K., Mallick, R., Tagalakis, V., Shivakumar, S., Schattner, A., Kuruvilla, P., Hill, D., Spadafora, S., Marquis, K., Trinkaus, M., Tomiak, A., Lee, A. Y. Y., Gross, P. L., Lazo-Langner, A., El-Maraghi, R., Goss, G., Le Gal, G., Stewart, D., … Wells, P.S. (2019). Apixaban to prevent venous thromboembolism in cancer patients. New England Journal of Medicine, 380(8), 711–719. https://doi.org/10.1056/nejmoa1814468
- PMID 30638566: Golemi I, Salazar Adum JP, Tafur A, Caprini J. Venous thromboembolism prophylaxis using the Caprini score. Disease-a-Month [Internet]. 2019;65(8):249–98. Available from: https://doi.org/10.1016/j.disamonth.2018.12.005
- PMID 30786186: Khorana, A. A., Soff, G. A., Kakkar, A. K., Vadhan-Raj, S., Riess, H., Wun, T., Streiff, M.B., Garcia, D. A., Liebman, H. A., Belani, C. P., O’Reilly, E.M., Patel, J. N., Yimer, H. A., Wildgoose, P., Burton, P., Vijapurkar, U., Kaul, S., Eikelboom, J., McBane, R., … Lyman, G. H. (2019). Rivaroxaban for thromboprophylaxis in high-risk outpatients with cancer. New England Journal of Medicine, 380(8), 720–728. https://doi.org/10.1056/nejmoa1814630
- PMID 31381464: Key, N. S., Khorana, A. A., Kuderer, N.M., Bohlke, K., Lee, A. Y. Y., Arcelus, J. I., Wong, S. L., Balaban, E. P., Flowers, C. R., Francis, C. W., Gates, L. E., Kakkar, A. K., Levine, M. N., Liebman, H. A., Tempero, M. A., Lyman, G. H., & Falanga, A. (2020). Prophylaxis and treatment of venous thromboembolism in patients with cancer: update of the clinical practice guideline of disgust. Journal of Clinical Oncology, 38(5), 496–520. https://doi.org/10.1200/JCO.19.01461
- PMID 31492632: Farge, D., Frere, C., Connors, J.M., Ay, C., Khorana, A. A., Munoz, A., Brenner, B., Kakkar, A., Rafii, H., Solymoss, S., Brilhante, D., Monreal, M., Bounameaux, H., Pabinger, I., Douketis, J., Ageno, W., Ajauro, F., Al-Aboudi, K. R., Alcindor, T., … Yamada, N. (2019). 2019 International Clinical Practice Guidelines for the Treatment and Prophylaxis of Venous Thromboembolism in Patients With Cancer. The Lancet Oncology, 20(10), e566–e581. https://doi.org/10.1016/S1470-2045(19)30336-5
- PMID 334649381: Vathiotis IA, Syrigos NK, Dimakakos EP. Tinzaparin Safety in Patients With Cancer and Renal Impairment: A Systematic Review. Clin Appl Thromb. 2021; 27:4–10.
- PMID 33861298: Osataphan, S., Patell, R., Chiasakul, T., Khorana, A. A. & Zwicker, J. I. (2021). Extended thromboprophylaxis for medically ill patients with cancer: a systemic review and meta-analysis. Blood Advances, 5(8), 2055–2062. https://doi.org/10.1182/BLOODADVANCES.2020004118
4. DISSEMINATED INTRAVASCULAR COAGULATION (DIC) AND MALIGNACY
Author: Soraia Marques Carvalho, MD
Co-author: Catarina Almeida,MD
Co-author: Alexandre Sarmento,MD
Co-author: Lúcia Borges, MD
Co-author: Mariana Texeira, MD
4.1 Backgroud
Disseminated intravascular coagulation (DIC) is a clinicopathological syndrome that represents an uncontrolled and potentially lethal hemostatic response to persistent systemic cellular damage that can be due to multiple causes, the most frequent being infection, trauma, and neoplasia.
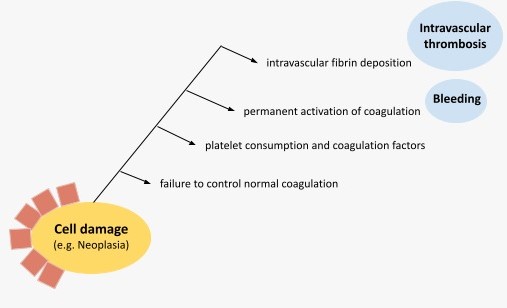
This condition is characterized by a sustained generation of thrombin that overwhelms the regulatory mechanisms of coagulation and leads to the intravascular deposition of fibrin and the development of microvascular thrombosis, known as thrombotic microangiopathy.
This leads, on the one hand, to ischemia and organ dysfunction (multiorgan dysfunction syndrome (MODS) and, on the other, to an excessive consumption of platelets and coagulation factors that favors bleeding (consumption coagulopathy). This latter scenario is particularly relevant if the fibrinolytic response is responsible for an accelerated plasmin generation, which leads to a clinical situation of hyperfibrinolysis.
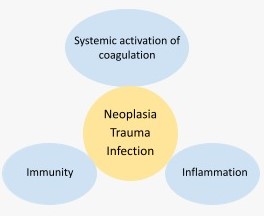
All the hemodynamic and metabolic alterations observed in DIC, are not only due to the generalized and/or persistent activation of coagulation, but also to the simultaneous activation of inflammation, innate immunity, and to the multiple interrelationships and feedback mechanisms that exist between all these systems (Fig.1).[1-4]
Click to see image
Fig.1. Relationship between the physiological hemostatic response, immunity, and inflammation.
This concept that leads to the notion of defibrination and consequent hemorrhagic state was introduced by Lasch y cols. in 1961 and was unified by the Scientific Standardization Committee of the International Society on Thrombosis and Haemostasis (SSC-ISTH).
4.2 Definition
DIC is an acquired syndrome characterized by activation of coagulation pathways, resulting in formation of intravascular thrombi and depletion of platelets and coagulation factors. [5,6] In a situation of malignancy, DIC is acquired/activated by the pre-existing condition
4.3 Aetiology
DIC is always a complication of an underlying disorder, and it can be triggered by multiple causes. In the presence of malignancy, it is triggered by exposure or release of procoagulant material into the bloodstream. [5,6]
As the most frequent causes of DIC in malignancy stand out acute promyelocytic leukemia, mucinous tumours (e.g., pancreatic, gastric, ovarian) and brain tumours.
Solid tumours (such as metastatic adenocarcinomas), chemotherapy, Tumour Lysis Syndrome (TLS), and Trousseau syndrome are strong risk factors in DIC development, and significant risk factors include age > 60 years, male sex, breast cancer, tumor necrosis, and advanced stage disease. [5,6,10]
4.4 Epidemiology
The occurrence of DIC varies considerably and is not directly comparable between the existing studies.
DIC is observed in patients with solid tumors and in a significant number of patients with hematological cancers, most often in acute leukemia, particularly acute promyelocytic leukemia.
The risk of incidence increases with several factors namely advanced-stage malignancies, thrombotic events and chemotherapy treatments.
Based on available data, it is estimated that the presence of malignant disease leads to a fourfold bigger risk of thrombosis, which rises to 6.5 when a patient receives chemotherapy.[6]
The clinical course depends on several factors such as age, presence of comorbidities, identification and treatment of underlying aetiologies, initial treatment response and severity of organ dysfunction, including the degree of haemostatic abnormalities.
While the incidence of sepsis-associated DIC seems stationary or decreasing over time, in-hospital survival did not improve for DIC accompanying solid tumors, hematological cancers, or other diseases.[11]
In recent years, the trends of incidence or mortality of DIC is unclear, therefore it is essential to carry out further studies.
4.5 Prognosis
DIC is a devastating condition with a poor prognosis.
4.6 Pathophysiology
Regardless of the underlying condition, the pathophysiology of DIC rests on 4 physiological mechanisms-elements: the increased tissue factor (TF) activity, the role of platelets, the overall loss of anticoagulant activity, and the altered fibrinolysis (Fig.2).
Click to see image
Fig.2. Pathophysiological scheme of DIC in malignancy.
4.7 Increased tissue factor activity and thrombin generation
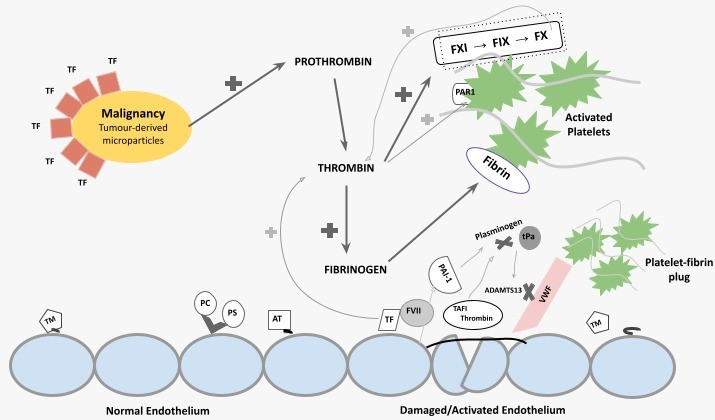
Tissue factor (TF) and possibly cancer procoagulant (CP), both expressed on the surface of malignant cells, through the activations of coagulation factor (F) VII and FX, increased TF activity and leads to thrombin generation (Fig.3).
This initially confers a prothrombotic state, as thrombin induces platelet activation, amplification of the coagulation cascade through positive feedback, and fibrin formation.
As DIC evolves, consumption of coagulation factors and platelets leads to hypo-coagulability, and subsequently an increased bleeding tendency. [12,13]
4.8 The role of Platelets
The cross-talk between tumor cells and platelets induces platelet activation in various cancer types, through the interaction with activated endothelium and the direct action of thrombin on platelets. The resulting platelet aggregation in the microcirculation leads to microthrombus formation and promotes pro-coagulant activity. Activated platelets present a negatively charged phospholipid surface where coagulation propagates through activation of FXI, FIX and FX.[14-16]
4.9 Loss of regulation
Under normal physiological circumstances, the endothelial surface enhances the effect of endogenous anticoagulant proteins that allow for normal hemostasis.
In malignancy, an inflammatory or in some cases trauma stimuli lead to endothelial disruption and increased vascular permeability. Endothelial anticoagulant surface proteins and glycans are shed, and endogenous anticoagulant activity is further impaired due to decreased production and increased turnover of antithrombin, protein C and protein S. VWF release is increased while simultaneously ADAMTS13 levels decrease. This further enhances platelet adhesion at the endothelial surface and contributes to microthrombus formation.[17]
Radio and chemotherapy treatments that are essential in most part of the therapeutic approach of malignancies cause endothelial cell disruption, providing of a suitable surface for the assembly of a platelet-fibrin clot.[7]
4.10 Altered fibrinolysis
Disturbed fibrinolysis is an important contributor to the pathophysiology of DIC. Fibrinolysis can be either impaired (hypofibrinolysis) or enhanced (hyperfibrinolysis) according to the underlying disease.
The impaired fibrinolysis is typical of sepsis and final stages of trauma while the enhanced fibrinolysis relates to malignant states and initial stages in trauma patients.
A malignant state is often associated with increased fibrinolytic activity usually secondary to increased pro-coagulant activity and fibrin formation, which in turns leads to increased plasminogen activation on the malignant cell surface and a consequent increase in plasmin activity.
Click to see image
Fig.3. Schematic summary of pathophysiology of DIC in malignancy.
TF, tissue factor; F, coagulation factor; PAR1, protease-activated receptor 1; TM, thrombomodulin; PC, protein C; PS, protein S; AT, antithrombin; PAI-1, plasminogen activator inhibitor-1; tPa, tissue plasminogen activator; TAFI, thrombin-activatable fibrinolysis inhibitor.
Hyperfibrinolysis is a common feature of acute promyelocytic leukemia and solid tumors, such as prostate cancer. In these conditions the patients often present with severe hyperfibrinolysis and bleeding.
For practical purposes there are three types of malignancy related DIC: procoagulant, hyperfibrinolytic and subclinical.
● Procoagulant DIC is distinguished by the excess thrombin generation that causes thrombosis in micro and macrovascular fields.
● In a Hyperfibrinolytic DIC the activation of the fibrinolytic system dominates the picture.
● In the Subclinical DIC the amount of thrombin and plasmin generated do not cause obvious clinical manifestations but is reflected in laboratory markers of coagulation or fibrinolysis activation.[10]
4.11 Clinical manifestations
The clinical presentation of DIC is highly variable and depends on the dynamic balance between clot formation in the microvasculature and degree of consumption of coagulation factors, inhibitors, and platelets.[8]
Clinical courses are typically less intense compared with DIC caused by sepsis/severe infection or major trauma.[6]
DIC in patients with malignancies include haemorrhagic complications, thrombosis of large or mid-sized vessels, thrombotic microangiopathy, or a combination of these, often manifested with insidious and protracted clinical symptoms of platelet and clotting factors consumption. Affected patients may be fully non-symptomatic and only detected by abnormalities in laboratory tests (Subclinical DIC). [6-8]
The ongoing consumption may result in bleeding complications (Hyperfibrinolytic DIC), usually the first clinical manifestation of DIC, frequently localized at the site of the tumor or distant metastases.[7] In this context, hemorrhage should serve as a red flag since it is the leading cause of mortality.
An alternative clinical scenario is dominated by thrombotic complications (Procoagulant DIC), ranging from clinically manifest vascular thrombosis to microvascular platelet plugs. [6,8,9]
Hematological cancers (promyelocytic or monocytic leukemia) are frequently accompanied by severe bleeding, whereas thrombotic complications are typical of solid cancers, especially adenocarcinomas, including prostate cancer, pancreas tumors, or other gastrointestinal malignancies. [7-10]
4.12 Diagnosis
Diagnosis is clinical and based on laboratory findings (Table 1).
In patients with cancer and DIC, abnormal coagulation tests are quite common. [5-7,10]
Platelet count, prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), fibrinogen and D-dimer/fibrin degradation products (FDPs) must be ordered to all suspected DIC patients, during initial assessment and monitoring (Table 1).
In some cases, all the typical coagulation abnormalities related to DIC, are present, whereas others will only display a moderately decreased platelet count or nearly normal clotting assay results due to adequate compensation of the consumed platelets and coagulation factors. [6,9,10]
Often a low platelet count is the most prominent indicator of DIC, due to an increase of clotting protein synthesis that camouflages the constant consumption of clotting proteins. [9,10]
Imaging studies or other tests must be ordered depending on the underlying disorder and location of thrombosis or bleeding.[5]
Dynamic viscoelastic point-of-care tests [thromboelastography (TEG ®) or thromboelastometry (ROTEM ®)] are laboratory methods with promising potential for diagnostic workup and prognosis. They are especially sensitive to hyper-fibrinolisis and hypo-coagulability and are mainly designed to detect severe coagulation disturbances and guide treatment in the bleeding patient.
Modified viscoelastic assays with added tissue plasminogen activator (tPA) are potentially more sensitive to hyper and hypo fibrinolysis but warrants further confirmation.[10]
Other emerging tests may be useful in the near future.[9]
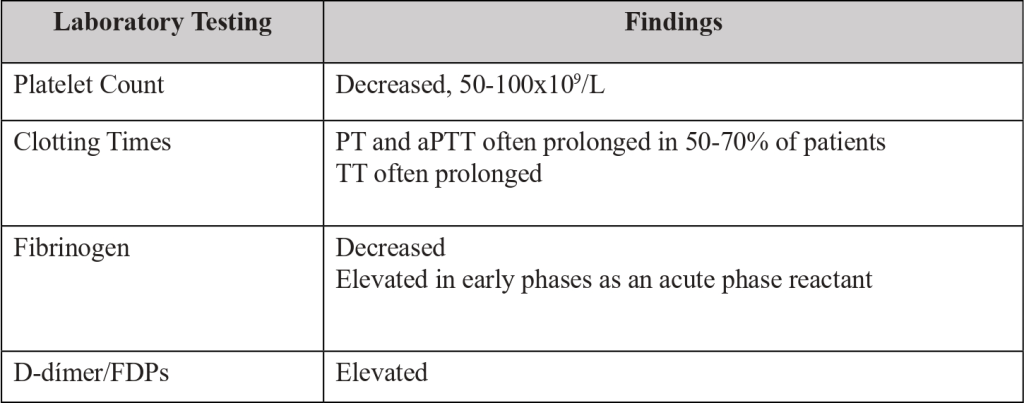
Table 1. Laboratory testing, utility, and usual results.
There are no validated scoring algorithms for DIC in cancer patients.
The Japanese Association for Acute Medicine (JAAM) DIC scoring algorithm may be useful in establishing a correct diagnosis, especially in patients with both solid tumors and hematological malignancies. [5,6] The JAAM DIC scoring algorithm includes a number of variables: criteria of systemic inflammatory response syndrome (SIRS), platelet count, FDPs, fibrinogen and PT. JAAM DIC Score ≥ 4 supports a diagnosis of DIC.
The scoring system of the International Society of Thrombosis and Hemostasis (ISTH) is more specific for CID caused by sepsis.[5]
4.13 Studies
The available data must be analyzed individually due to the excess of variables involved.
One study including 204 DIC patients showed that patients with DIC related malignancy were more likely to develop clinically significant bleeding (30–50%, depending on cancer type) than sepsis-related DIC, where bleeding was less common (15%) and often a late manifestation. In sepsis-related DIC, organ dysfunction indicating microthrombus was predominant (70%), while was less pronounced in cancer patients.[10]
4.14 Therapeutic Strategy
The management of DIC is complex (Table 2).
The main principle of DIC management in malignancy is adequate treatment of the precipitating disorder.
There are clinical presentations that may require additional supportive strategies specifically aimed at the amelioration of the coagulopathy. [6,9,11]
Most of the therapeutic measures are surprisingly not based on high levels of evidence.[11]
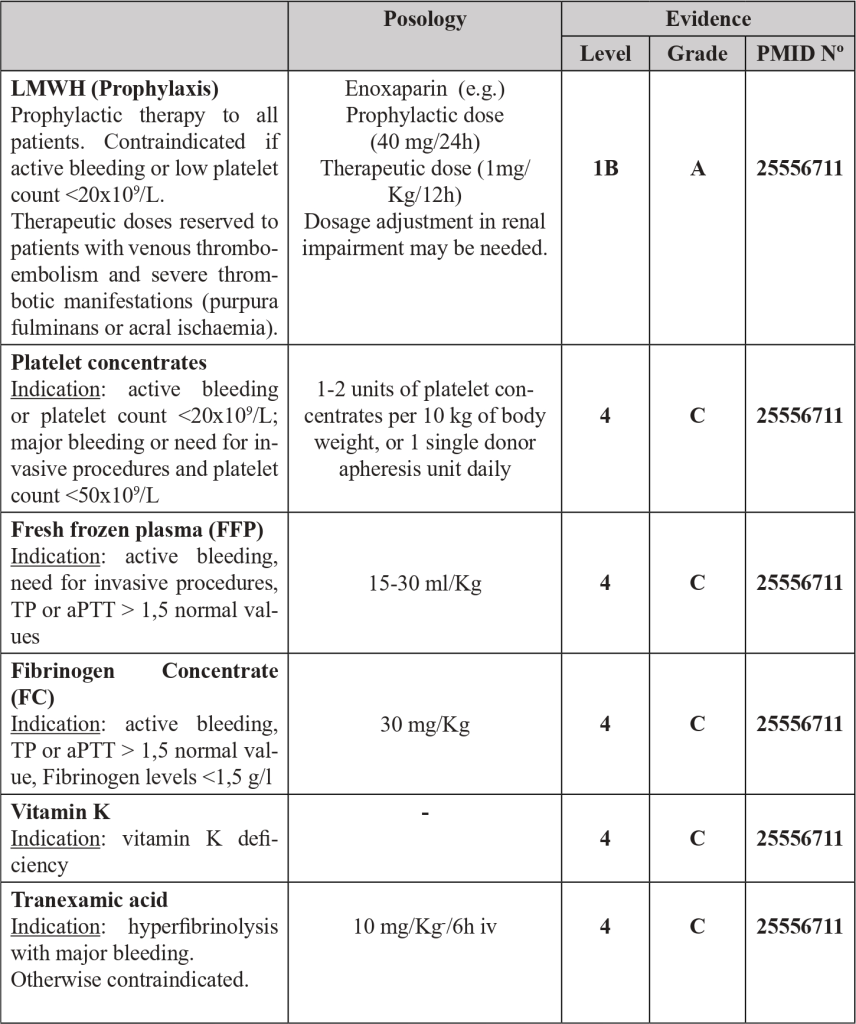
4.15 Malignancy related DIC sum up
Each malignancy related DIC has their distinctive features, mechanisms and clinical presentation that invariably determine and restrict their respective therapeutic approach (Table 3).
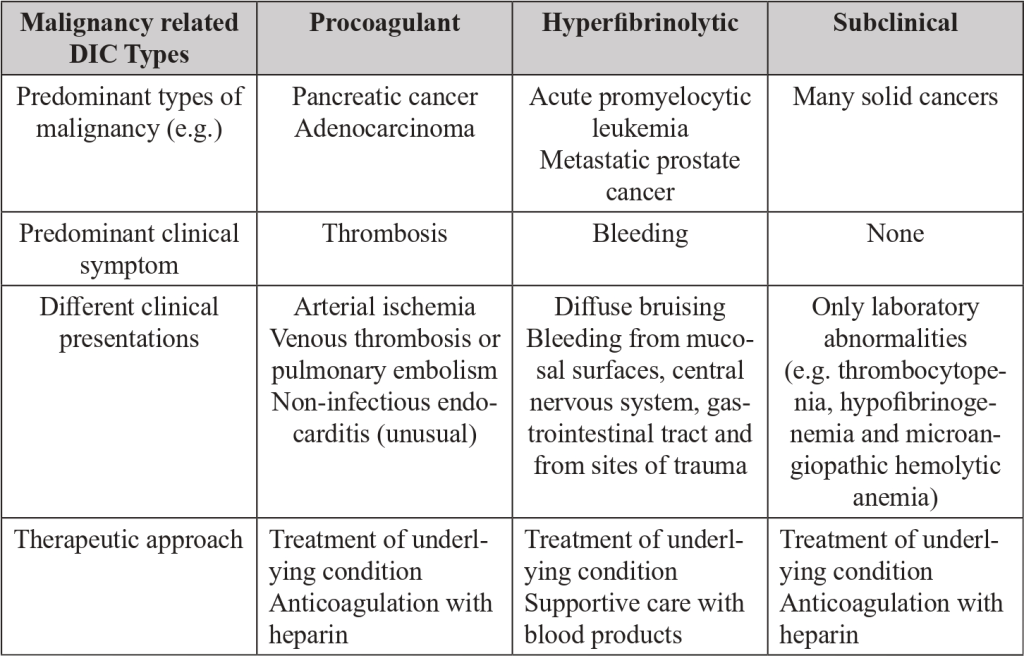
Table 3. Malignancy related DIC sum up. Distinctive features of procoagulant, hyperfibrinolytic and subclinical DIC.
4.16 Key points
● DIC is characterized by systemic intravascular coagulation activation, leading to deposition of intravascular platelets and fibrin, and simultaneous consumption of coagulation proteins and thrombocytes.
● TF and CP, both expressed on malignant cells, can initiate the activation of coagulation.
● A disturbed physiological anticoagulant pathway and unbalanced fibrinolysis also play a pivotal role.
● Haemorrhagic complications and thrombosis of large or mid-sized vessels are the main clinical manifestations.
● The malignancy related DIC may evolve through time changing their mechanism and clinical presentation.
● The hyperfibrinolytic DIC is the most challenging medical approach as hemorrhage is the most common cause of mortality.
● Diagnosis is clinical and based on abnormal coagulation tests.
● The therapeutic approach is complex, based on the treatment of underlying malignancy and supportive treatment, with anticoagulation and blood products as needed
References
1. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009; 145: 24-33.
2. Toh CH, Alhamdi Y. Current consideration and management of disseminated intravascular coagulation. Hematology Am Soc Hematol Educ Program. 2013; 2013: 286-91.
3. Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med.1999; 341: 586-92.
4. Vincent JL, De Backer D. Does disseminated intravascular coagulation lead to multiple organ failure? Crit Care Clin. 2005; 21: 469-77.
5. Wang, H. (2021). Disseminated intravascular coagulation Straight to the point of care. BMJ Best Practice.
6. Levi, M. (2019). Disseminated intravascular coagulation in cancer: An update. Seminars in Thrombosis and Hemostasis, 45(4), 342–347.
7. Feinstein DI. Disseminated intravascular coagulation in patients with solid tumors. Oncology (Williston Park) 2015;29(02):96–102.
8. Levi M. Clinical characteristics of disseminated intravascular coagulation in patients with solid and hematological cancers. Thromb Res 2018;164(Suppl 1):S77–S81.
9. Wada H, Thachil J, Di Nisio M, et al; The Scientific Standardization Committee on DIC of the International Society on Thrombosis Haemostasis. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost 2013.
10. Sallah S, Wan JY, Nguyen NP, et al. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost 2001; 86:828.
11. Adelborg, K., Larsen, J. B., & Hvas, A. M. (2021). Disseminated intravascular coagulation: epidemiology, biomarkers, and management. In British Journal of Haematology (Vol. 192, Issue 5, pp. 803–818). Blackwell Publishing Ltd.
12. Dicke C, Amirkhosravi A, Spath B, Jimenez-Alcazar M, Fuchs T, Davila M, et al. Tissue factor-dependent and -independent pathways of systemic coagulation activation in acute myeloid leukemia: a single-center cohort study. Exp Hematol Oncol. 2015;4:22.
13. Thaler J, Koder S, Kornek G, Pabinger I, Ay C. Microparticle-associated tissue factor activity in patients with metastatic pancreatic cancer and its effect on fibrin clot formation. Transl Res. 2014;163:145–50.
14. Laursen MA, Larsen JB, Hvas AM. Platelet function in disseminated intravascular coagulation: a systematic review. Platelets. 2018;29:238–48.
15. Wang Y, Ouyang Y, Liu B, Ma X, Ding R. Platelet activation and antiplatelet therapy in sepsis: a narrative review. Thromb Res. 2018;166:28–36.
16. Hoffman M. A cell-based model of coagulation and the role of factor VIIa. Blood Rev. 2003;17(Suppl 1):S1–S5.
17. Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037.
5. BLEEDING IN CANCER PATIENT
Author: José Pedro Cidade, MD
Co-author: Tânia Duarte, MD
Co-author: Rehab Ahmed Hamdy, MD
5.1 Introduction
1PMID 15477642, 2PMID 31006890, 3PMID 19347728, 4PMID 3308824
Bleeding in cancer patients can occur from chronic occult bleeding to clinically significant macroscopic bleeding or profound bleeding from large blood vessels which may cause sudden death1. It can be the first symptom or develop later along with disease progression. It has been estimated that bleeding occurs in approximately 6-10% of patients with advanced cancer; for at least some of these patients, bleeding will be the direct cause of death.
5.2 Aetiology
3PMID 19347728, 4PMID 3308824, 5PMID 35579752
It is universally accepted that cancer patients present an increased risk of bleeding, and that risk is multifactorial in its aetiology, potentially attributed to several factors:
1. Local infiltration of blood vessels by tumour: There may be anatomical or radiographic signs of tumour near a major blood vessel where direct infiltration can lead to a sudden bleed. Warning signs of visible pulsations in malignant wounds, or a sudden increase in pain should prompt a swift assessment of the patient.
2. Cancer treatments such as radiotherapy, chemotherapy, or surgery: Chemoradiotherapy-induced myelosuppression commonly manifests as thrombocytopenia and often results in increased risk of bleeding. In addition, newer agents such as Bevacizumab have direct effects on tumour angiogenesis, with recognised complications of bowel perforation and delayed healing after surgery. Local inflammation around surgery or radiotherapy sites also results in an increased risk of bleeding.
3. Systemic complications of cancer: Liver disease, biliary obstruction or bowel problems can lead to deficiencies in clotting factors and an increased bleeding tendency. Some patients may have an underlying coagulopathy due to the illness itself. Disseminated Intravascular Coagulation (DIC) is seen in many forms of cancer. Thrombocytopenia and platelet dysfunction is also commonly seen in haematological malignancies and other condition such as thrombotic thrombocytopenic purpura due to cancer or chemotherapy.
4. Drug treatments such as anticoagulants or non-steroidal anti-inflammatory agents: There are many drugs that interfere with platelet function (such as aspirin, clopidogrel), usually prescribed to patients with tumours. The use of anticoagulants such as warfarin and low molecular weight heparin will also increase bleeding risk.
5. Concurrent illness, including infection: Local infection within tumour cavities can also increase the risk of bleeding. If an infection is suspected antibiotic therapy should be considered to reduce this risk.
5.3 Symptoms
6PMID 29307210, 7PMID 3382303
Bleeding is a frequent problem for patients with advanced cancer, with approximately 10% of all patients having at least one episode and almost 30% in patients with hematologic malignancies (2). These episodes may range from low-grade oozing to major episodic bleeding or even catastrophic bleeds. Patients may develop acute catastrophic bleeding, episodic major bleeding, or low-volume oozing. Bleeding may present as bruising, petechiae, epistaxis, haemoptysis, hematemesis, haematochezia, melena, haematuria, or vaginal bleeding.
5.4 Diagnosis/Assessment
6PMID 29307210
In the event of severe haemorrhage, or if the risk of a bleed is thought to be significant, a decision should be made regarding the most appropriate place of care for the patient. A multidisciplinary discussion may be needed to reach an informed decision about possible treatment options. Facilities for highly specialised interventions (e.g., specialist surgery, radiotherapy, or interventional radiology) may not be available in smaller centres. So, the decisions about what is required to manage a patient’s bleeding problem should be made at an early stage.
General approach to the management of bleeding in cancer patients
The following steps are a suggested means of assessing a cancer patient who presents with bleeding. It should only be used as a guide, and should not undermine a patient-tailored approach:
1. Where is/are the site(s) of bleeding?
a. For external bleeding: apply a dressing to reduce bleeding and protect the wound from trauma and infection.
2. How large is the bleed?
The following should be considered:
a. Pulse, lying and standing BP (a postural blood pressure drop is often the first sign of blood loss)
b. Blood analysis with complete blood count, coagulation profile and a metabolic panel should are mandatory (to check for dehydration; also a disproportionately high urea may suggest a gastrointestinal bleeding source). Consider securing IV access at this point.
c. Fluid resuscitation to maintain blood pressure and vital organ perfusion. Indications may include a positive shock index characterized by a pulse rate (in beats per minute) greater than the systolic blood pressure (in mmHg).
d. In a patient with haemorrhage symptoms and suspicion of Disseminated Intravascular Coagulation (DIC), proceed to calculation of DIC score. A score above 7 should prompt the consideration of immediate DIC treatment.
3. Are there any reversible local or systemic causes?
a. Review medications and consider stopping any drugs that adversely affect clotting. For patients on anticoagulant drugs for deep venous thrombosis (DVT) or a pulmonary embolus (PE), an overall assessment of likely risks versus benefit on continuing treatment needs to be made and ideally communicated with the patient.
b. Where there appears to be bleeding from several different sites, consider an underlying coagulopathy and whether this should be corrected (may need to consult a local haematologist for advice).
c. If infection is thought to precipitate haemorrhage, consider wound swabs and cultures for microbiological identification of pathogens and antimicrobial sensitivities
4. Are there any immediate local measures that can be used?
5. Decide on most appropriate place of care for the patient, both now and in the event of deterioration.
6. Regularly review the treatment plan and ensure that planned management is documented and communicated clearly to all staff involved in the patient’s treatment.
Analysis should include a complete blood count, coagulation profile, and a complete metabolic panel with assessment of liver enzymes and function. It may be useful to perform imaging studies including computed tomography or angiography of the area suspected of bleeding, and/or endoscopy. Possible contributing factors including comorbidities, medications, and recent therapeutic interventions should be examined. If the patient is on anticoagulation therapy, the risks of further bleeding versus those of clotting should be examined and discussed. Use of oral anticoagulants has been associated with genitourinary cancer in atrial fibrillation patients with haematuria, so it is important to consider stopping it, and to carefully evaluate these patients for the cause of haematuria.
5.5 Therapeutic Strategy
8PMID 24122413, 9PMID 10963635, 10PMID 22611164, 11PMID 11163503, 12PMID 8814371, 13PMID 9192963, 14PMID 21482082, 15PMID 15928300, 16PMID 20833516, 17PMID 8814372
Systemic Therapies
Local Therapies
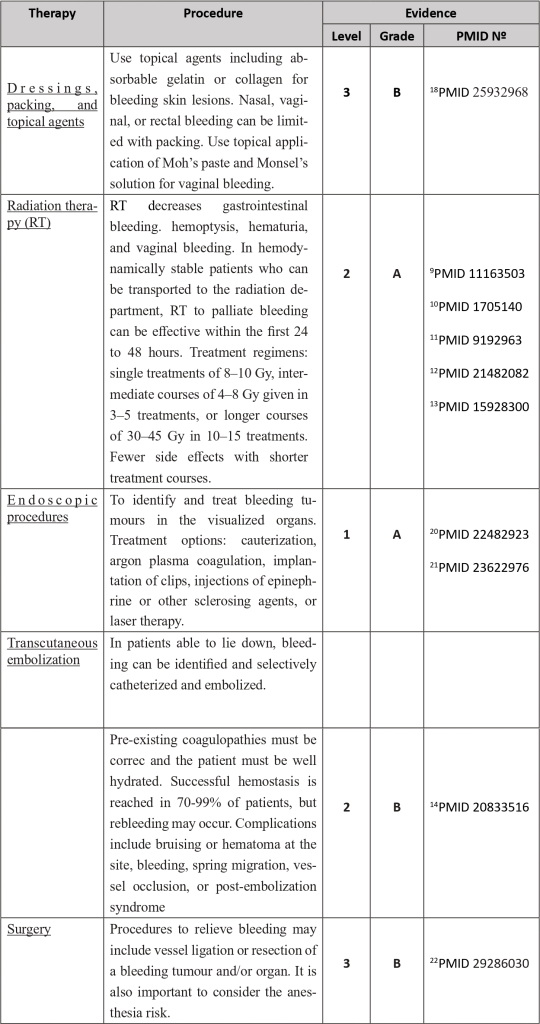
Treatment for selected sites of haemorrhage
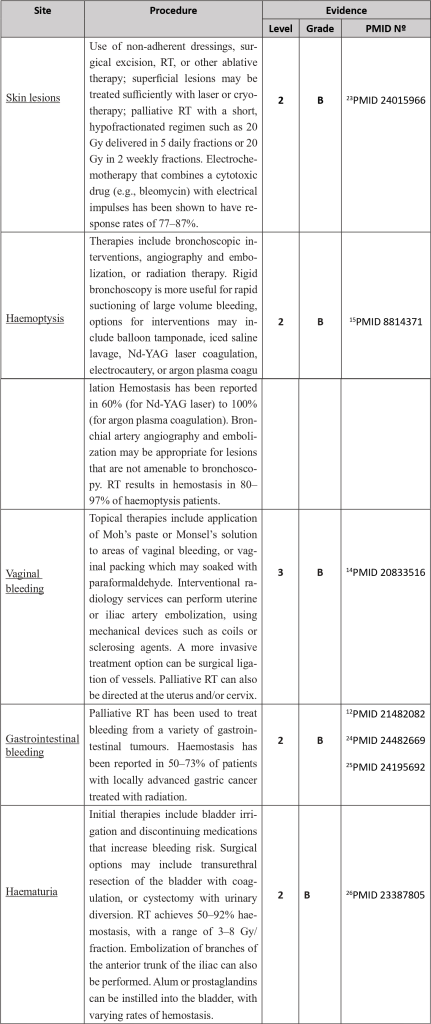
References
- Pereira J, Phan T: Management of bleeding in patients with advanced cancer. Oncologist 2004, 9: 561-570. 10.1634/theoncologist.9-5-561
- Angelini DE, Radivoyevitch T, McCrae KR, Khorana AA. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am J Hematol. 2019 Jul;94(7):780-785. doi: 10.1002/ajh.25494. Epub 2019 May 16. PMID: 31006890.
- Cartoni C, Niscola P, Breccia M, et al. Hemorrhagic complications in patients with advanced hematological malignancies followed at home: An Italian experience. Leuk Lymphoma 2009;50:387-91
- JP Dutcher. Hematologic abnormalities in patients with nonhematologic malignancies. Hematol Oncol Clin North Am 1987; 1: 281–299.
- Escobar A, Salem AM, Dickson K, Johnson TN, Burk KJ, Bashoura L, Faiz SA. Anticoagulation and bleeding in the cancer patient. Support Care Cancer. 2022 Oct;30(10):8547-8557. doi: 10.1007/s00520-022-07136-w. Epub 2022 May 17. PMID: 35579752.
- Johnstone C, Rich SE. Bleeding in cancer patients and its treatment: a review. Ann Palliat Med. 2018 Apr;7(2):265-273. doi: 10.21037/apm.2017.11.01. Epub 2017 Dec 18. PMID: 29307210.
- Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med 1988;148:1586-91.
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: The TEACHH model. Cancer 2014;120:134-41.
- Hutten BA, Prins MH, Gent M, et al. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: A retrospective analysis. J Clin Oncol 2000;18:3078-83
- Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
- Crane CH, Janjan NA, Abbruzzese JL, et al. Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys 2001;49:107-16.
- Inoperable non-small-cell lung cancer (NSCLC): A Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer 1991;63:265-70.
- McLaren DB, Morrey D, Mason MD. Hypofractionated radiotherapy for muscle invasive bladder cancer in the elderly. Radiother Oncol 1997;43:171-4.
- Yan J, Milosevic M, Fyles A, et al. A hypofractionated radiotherapy regimen (0-7-21) for advanced gynaecological cancer patients. Clin Oncol (R Coll Radiol) 2011;23:476-81.
- Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005;97:798-804.
- Hague J, Tippett R. Endovascular techniques in palliative care. Clin Oncol (R Coll Radiol) 2010;22:771-80.
- Macbeth FR, Bolger JJ, Hopwood P, et al. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:167-75.
- Eleje GU, Eke AC, Igberase GO, et al. Palliative interventions for controlling vaginal bleeding in advanced cervical cancer. Cochrane Database Syst Rev 2015;5:CD011000.
- Khatib R, Ludwikowska M, Witt DM, Ansell J, Clark NP, Holbrook A, Wiercioch W, Schünemann H, Nieuwlaat R. Vitamin K for reversal of excessive vitamin K antagonist anticoagulation: a systematic review and meta-analysis. Blood Adv. 2019 Mar 12;3(5):789-796. doi: 10.1182/bloodadvances.2018025163. PMID: 30850385; PMCID: PMC6418499.
- Chen YI, Barkun AN, Soulellis C, et al. Use of the endoscopically applied hemostatic powder TC-325 in cancer-related upper GI hemorrhage: Preliminary experience (with video). Gastrointest Endosc 2012;75:1278-81.
- Leblanc S, Vienne A, Dhooge M, et al. Early experience with a novel hemostatic powder used to treat upper GI bleeding related to malignancies or after therapeutic interventions (with videos). Gastrointest Endosc 2013;78:169-75.
- Shabunin AV, Bagateliya ZA, Korzheva IY, Lebedev SS. Neotlozhnaia khirurgicheskaia pomoshch’ bol’nym rakom tolstoĭ i priamoĭ kishki, oslozhnennym krovotecheniem [Urgent surgical care for patients with colon cancer complicated by hemorrhage]. Khirurgiia (Mosk). 2017;(12):46-51. Russian. doi: 10.17116/hirurgia20171246-51. PMID: 29286030.
- Kähler KC, Egberts F, Gutzmer R. Palliative treatment of skin metastases in dermato-oncology. J Dtsch Dermatol Ges. 2013 Nov;11(11):1041-5; quiz 1046. doi: 10.1111/ddg.12197. Epub 2013 Sep 9. PMID: 24015966.
- Chaw CL, Niblock PG, Chaw CS, et al. The role of palliative radiotherapy for haemostasis in unresectable gastric cancer: A single-institution experience. Ecancermedicalscience 2014;8:384
- Cameron MG, Kersten C, Vistad I, et al. Palliative pelvic radiotherapy of symptomatic incurable rectal cancer – a systematic review. Acta Oncol 2014;53:164-73.
- Abt D, Bywater M, Engeler DS, et al. Therapeutic options for intractable hematuria in advanced bladder cancer. Int J Urol 2013;20:651-60.
← BACK